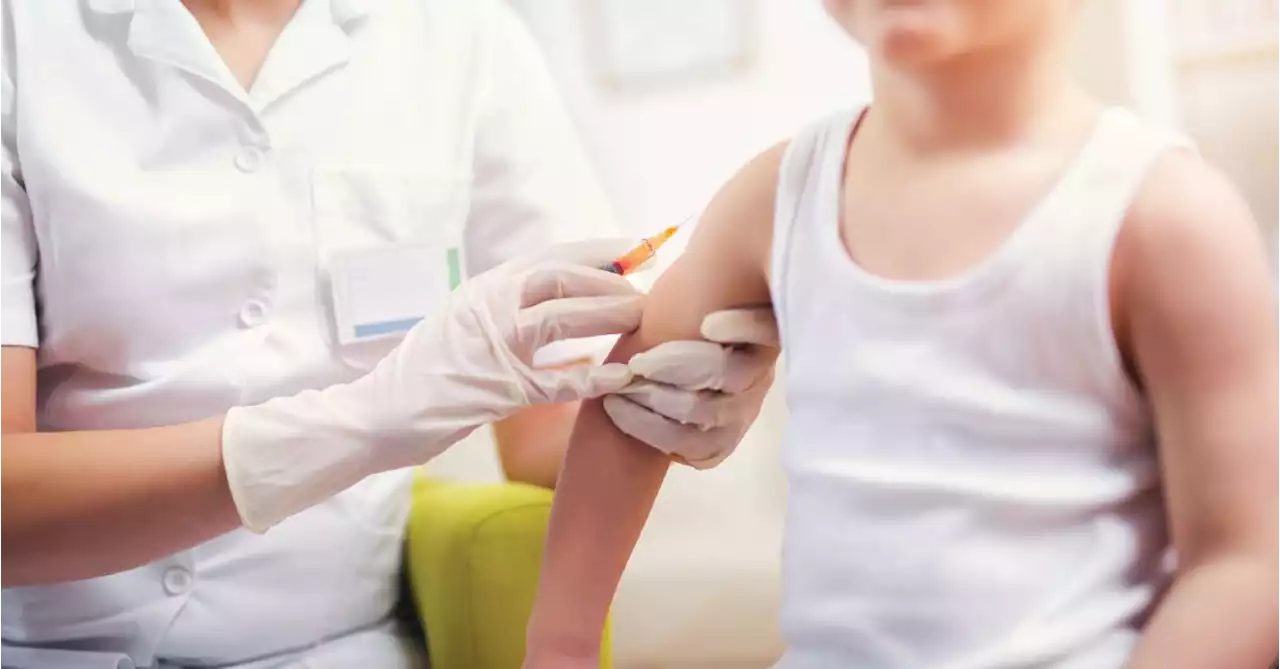
The FDA today granted emergency use authorization to the Moderna and Pfizer COVID-19 vaccines for use in children 6 months of age and older.
"The FDA’s evaluation and analysis of the safety, effectiveness, and manufacturing data of these vaccines was rigorous and comprehensive, supporting the EUAs," the agency said in aThe data shows that the “known and potential benefits” of the vaccines outweigh any potential risks, the agency said.
The Moderna vaccine is authorized as a two-dose primary series in children 6 months to 17 years of age. The Pfizer vaccine is now authorized as a three-dose primary series in children 6 months up to 4 years of age. Pfizer’s vaccine was already authorized in children 5 years old and older. Now all eyes are on the CDC, which is expected to decide on the final regulatory hurdle at a meeting Saturday. The CDC's Advisory Committee on Immunization Practices has scheduled a vote on whether to give the vaccines the green light.
If ACIP gives the OK, CDC Director Rochelle Walensky, MD, MPH, is expected to issue recommendations for use shortly thereafter. Following these final regulatory steps, parents could start bringing their children to pediatricians, family doctors, or local pharmacies for vaccination as early as Monday.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
FDA authorizes COVID vaccine for children under 5, CDC review nextAn FDA advisory panel unanimously found both vaccines to be “safe and effective” for children as young as 6 months old.
Read more »
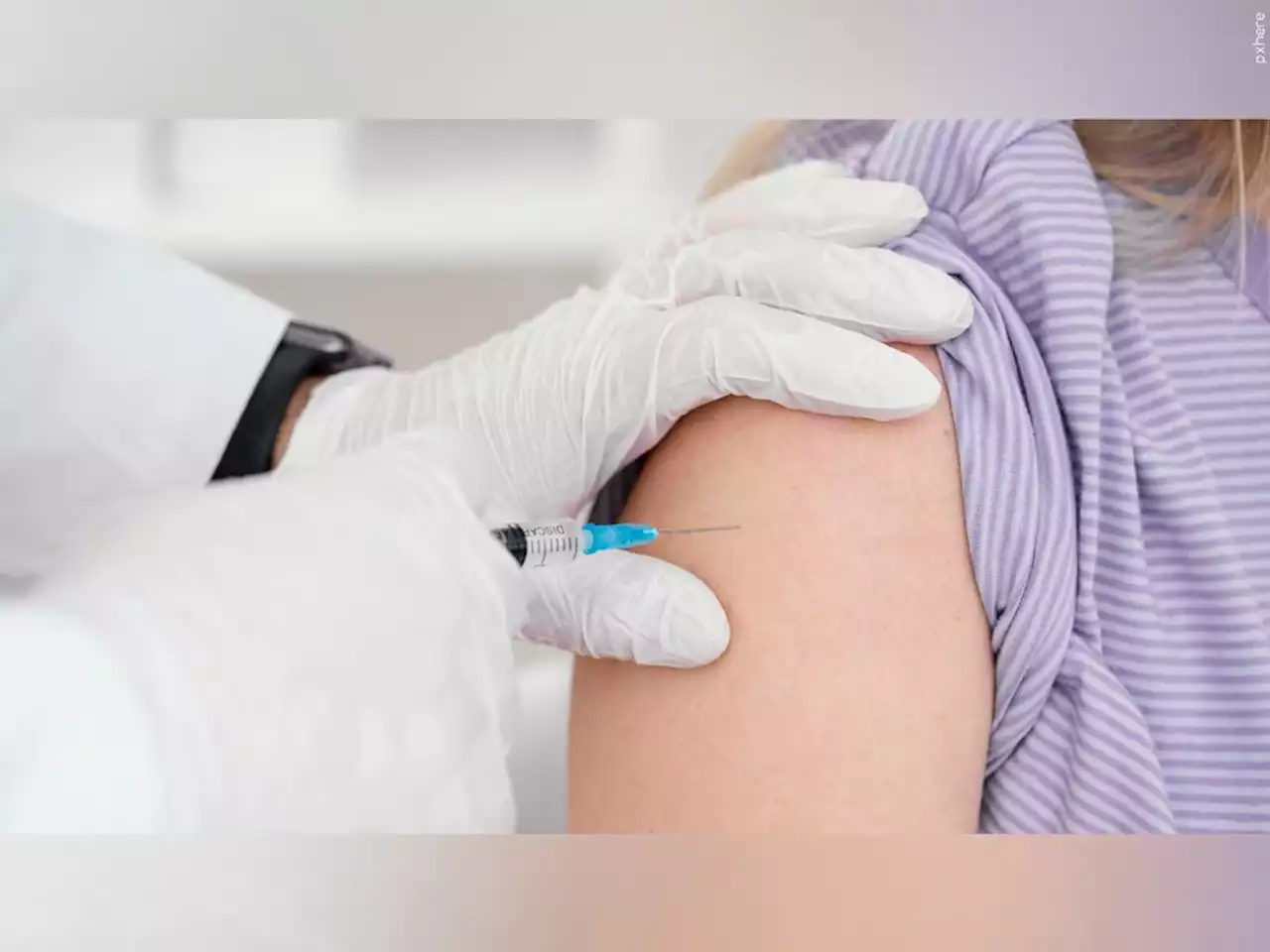 FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
FDA authorizes 1st COVID-19 shots for infants, preschoolersFDA authorizes 1st COVID-19 shots for infants, preschoolers.
Read more »
 FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
FDA Authorizes 1st COVID-19 Shots for Infants, PreschoolersJUST IN: The FDA has authorized the first COVID-19 shots for children under the age of five, paving the way for vaccinations to begin once CDC approves.
Read more »
 COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
COVID-19 vaccine: FDA authorizes 1st shots for kids under 5; CDC review nextBREAKING: U.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Read more »
 FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
FDA authorizes 1st COVID-19 shots for kids under 5; CDC review is nextU.S. regulators on Friday authorized the first COVID-19 shots for infants and preschoolers, paving the way for vaccinations to begin next week.
Read more »
 FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
FDA authorizes Pfizer, Moderna COVID-19 vaccines for kids under 5The FDA this morning authorized the first COVID-19 vaccines for children under 5, bringing shots for America's youngest children one step closer to reality.
Read more »