Unlike any other, it uses a nasal swab sample to test for COVID, flu and respiratory syncytial virus at the same time.
Those samples are then sent back to the test maker for testing and results are delivered through an online portal.Meanwhile, the American Academy of Pediatrics reported new COVID-19 cases among U.S. kids are up for the fifth week in a row.
The company said a third vaccine dose raised omicron-fighting antibodies by 36 times in this age group.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
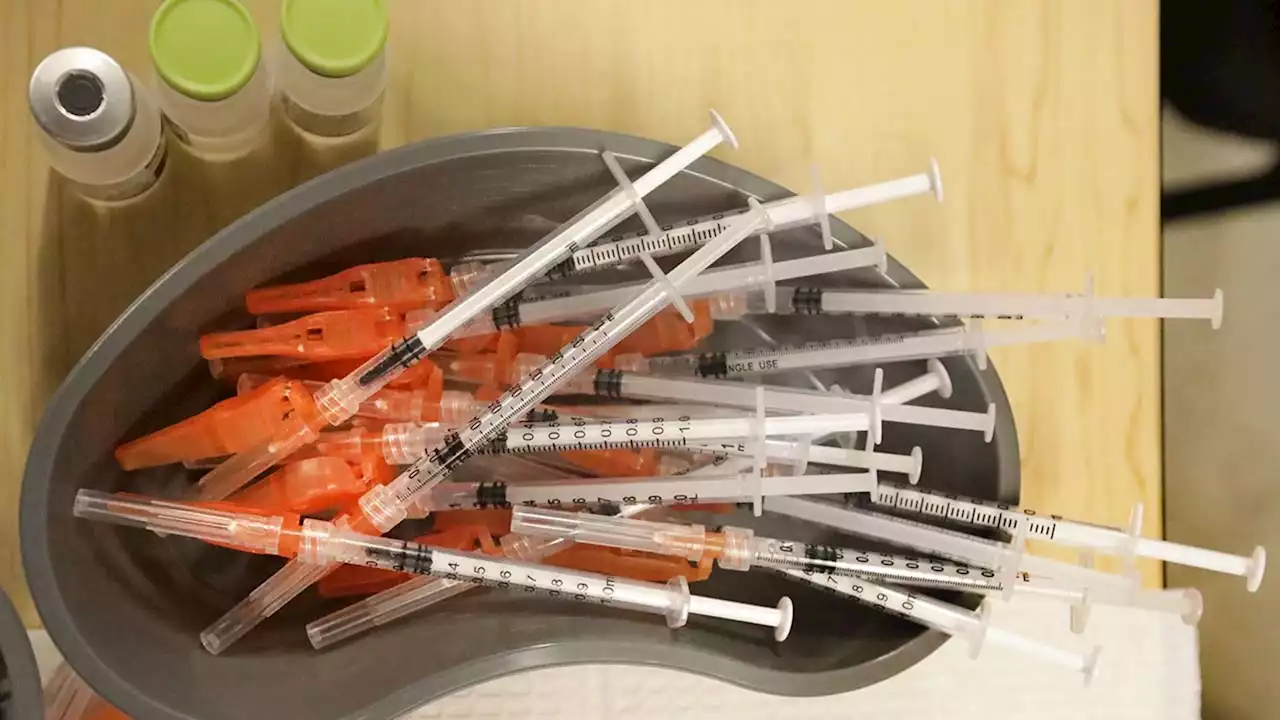 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA authorizes Pfizer COVID-19 booster for kids 5-11A rise in new COVID-19 cases among children was reported overnight by health leaders but there may be extra protection coming for those children in the form of booster doses of vaccines. Andrea Nakano reports. (5-17-22)
FDA authorizes Pfizer COVID-19 booster for kids 5-11A rise in new COVID-19 cases among children was reported overnight by health leaders but there may be extra protection coming for those children in the form of booster doses of vaccines. Andrea Nakano reports. (5-17-22)
Read more »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11 -The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech’s Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series. Pfizer requested this EUA at the end of April, citing company data that showed that a third vaccine dose raised Omicron-fighting antibodies by 36...
Read more »
