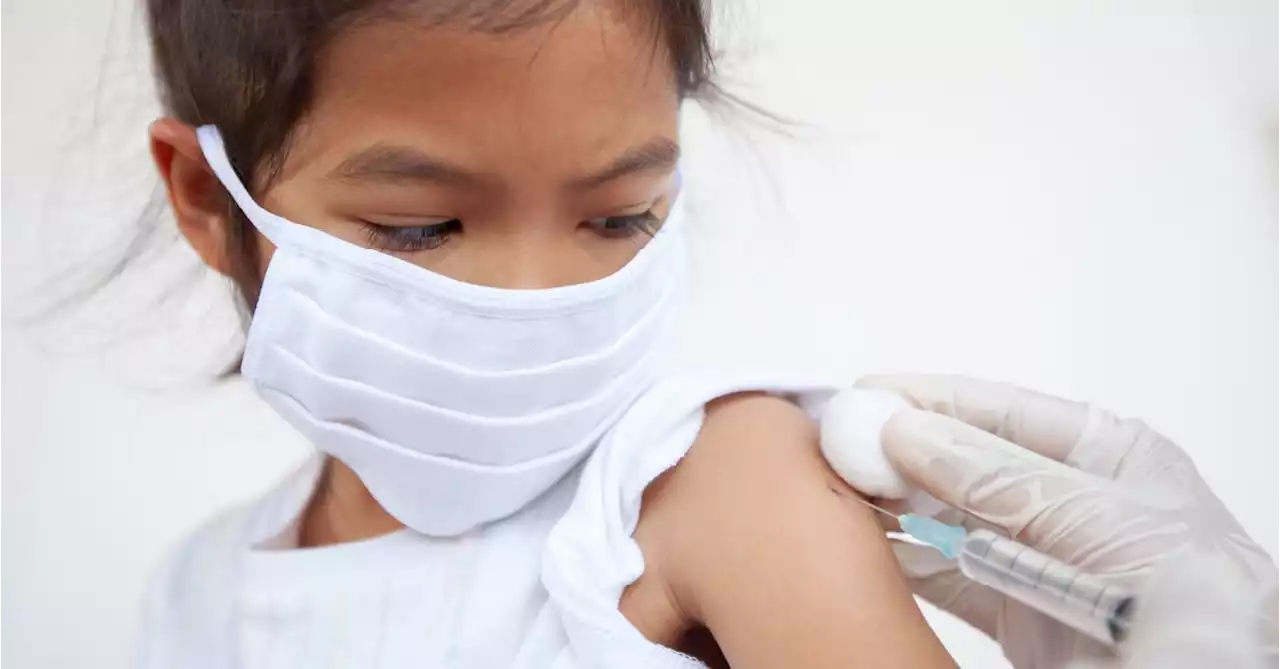
The FDA expanded an emergency use authorization (EUA) today, allowing the Pfizer-BioNTech COVID-19 booster shot for children ages 5 to 11 who are at least 5 months out from their first vaccine series.
for an amended authorization in April, after submitting data showing that a third dose in children between 5 and 11 raised antibodies targeting the Omicron variant by 36 times.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, the omicron wave has seen more kids getting sick with the disease and being hospitalized, and children may also experience longer-term effects, even following initially mild disease,” FDA Commissioner Robert M. Califf, MD, said in a news release.
To some experts, this data suggests an even greater need for children under 12 to be eligible for a third dose. “Since authorizing the vaccine for children down to 5 years of age in October 2021, emerging data suggest that vaccine effectiveness against COVID-19 wanes after the second dose of the vaccine in all authorized populations,” says Peter Marks, MD, PhD, the director of the FDA’s Center for Biologics Evaluation and Research.
The CDC still needs to sign off on the shots before they can be allowed. The agency’s Advisory Committee on Immunization Practices isto discuss allowing Pfizer’s and Moderna’s COVID-19 vaccines for children under 6 years old.Pfizer: “Pfizer and BioNTech Granted U.S. Emergency Use Authorization for Booster Dose of Their COVID-19 Vaccine in Children 5 Through 11 Years of Age.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
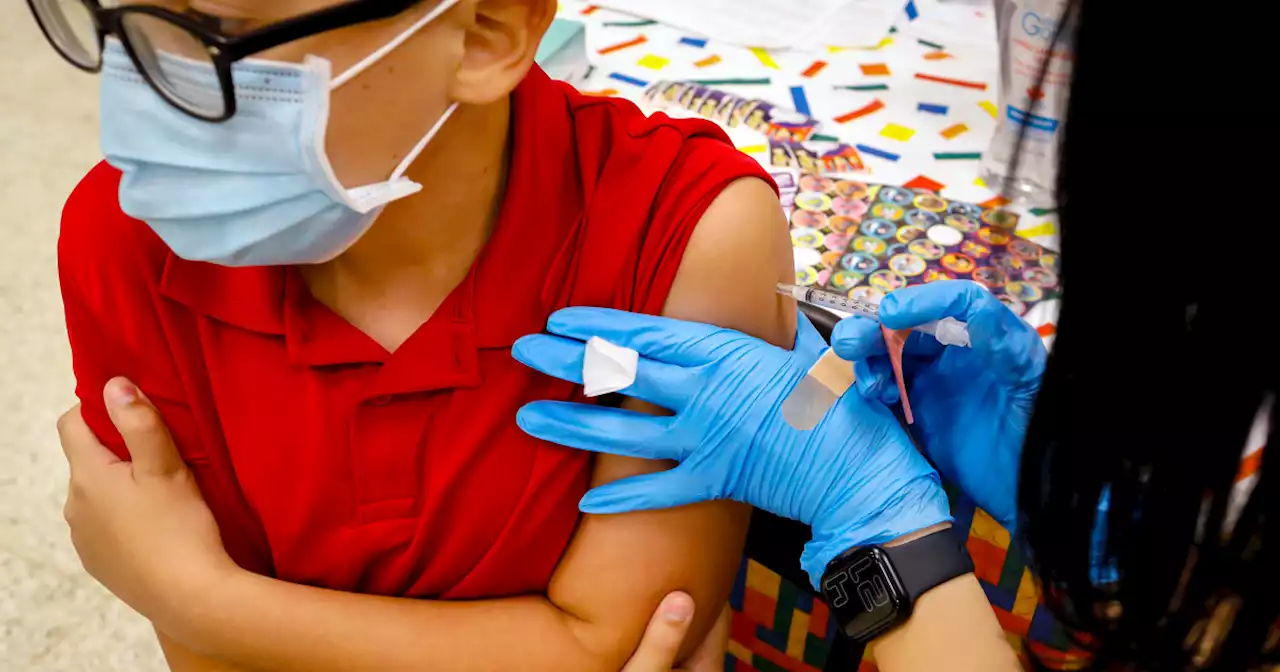 FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
FDA authorizes Pfizer Covid booster for children 5 to 11 years oldA study in February found that two doses of the vaccine offered little protection against the omicron variant.
Read more »
 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
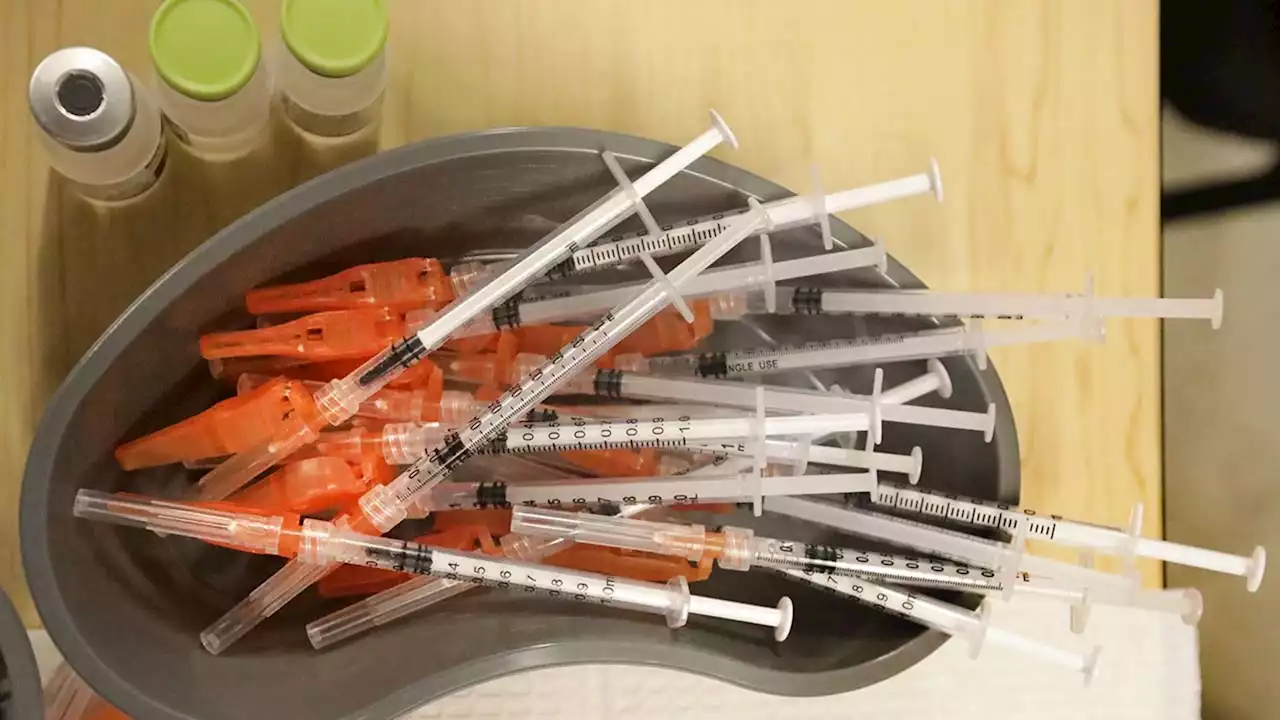 FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer COVID-19 booster shots for children ages 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
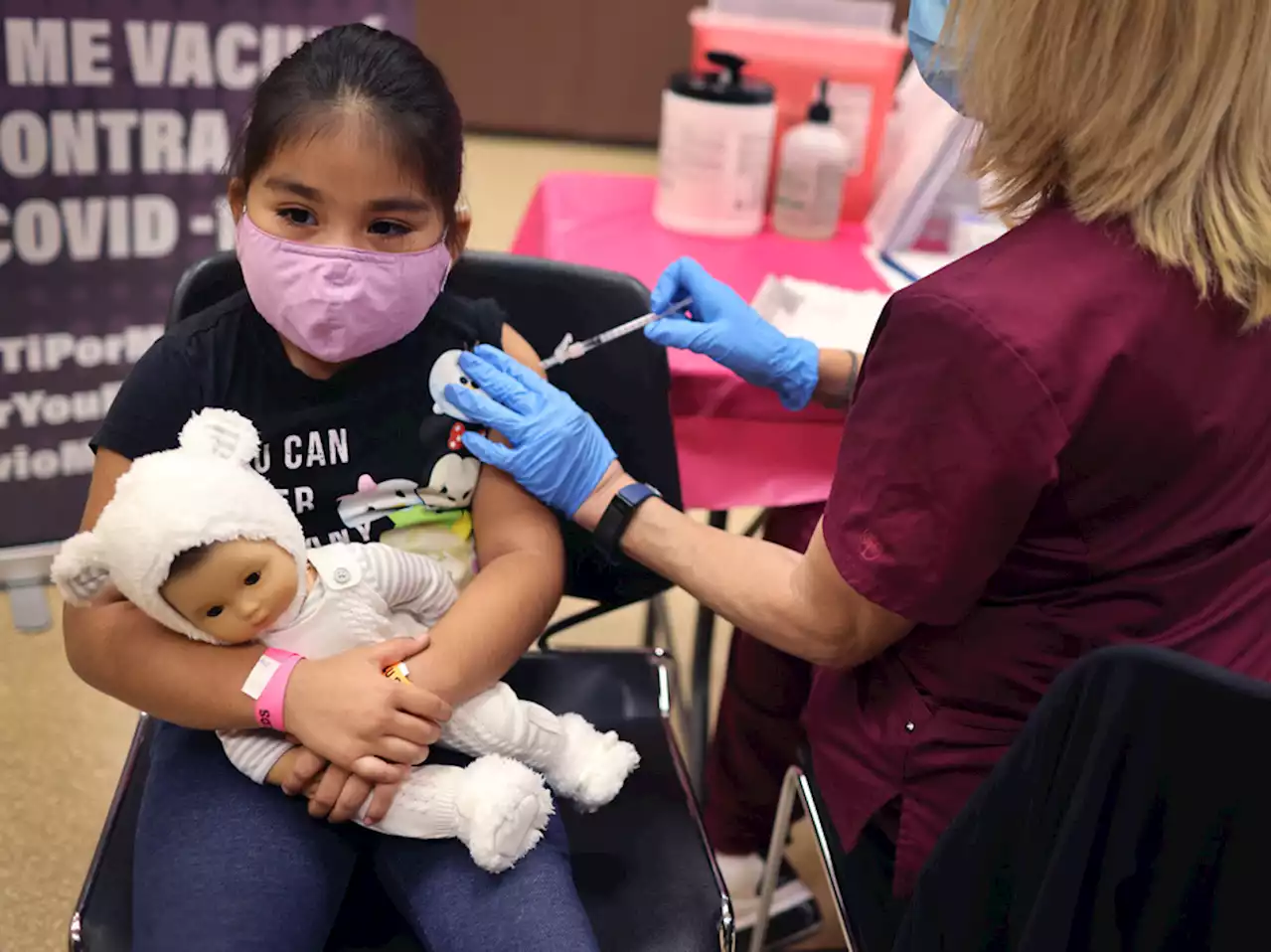 FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
Read more »
 FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
FDA Authorizes Pfizer Covid Booster Dose for Kids Ages 5 to 11 Years OldThe Food and Drug Administration on Tuesday authorized a Pfizer booster dose for children ages 5 through 11 years old at least five months after they complete their two-dose primary series. Dr. Peter Marks, head of the FDA division responsible for vaccines, said data increasingly shows that the protection provided by two shots wanes off over time. The FDA determined…
Read more »
 FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
FDA approves Pfizer COVID booster shots for kids 5 to 11The FDA has granted emergency use authorization for a booster dose of Pfizer/BioNTech's COVID-19 vaccine for children ages 5 to 11.
Read more »