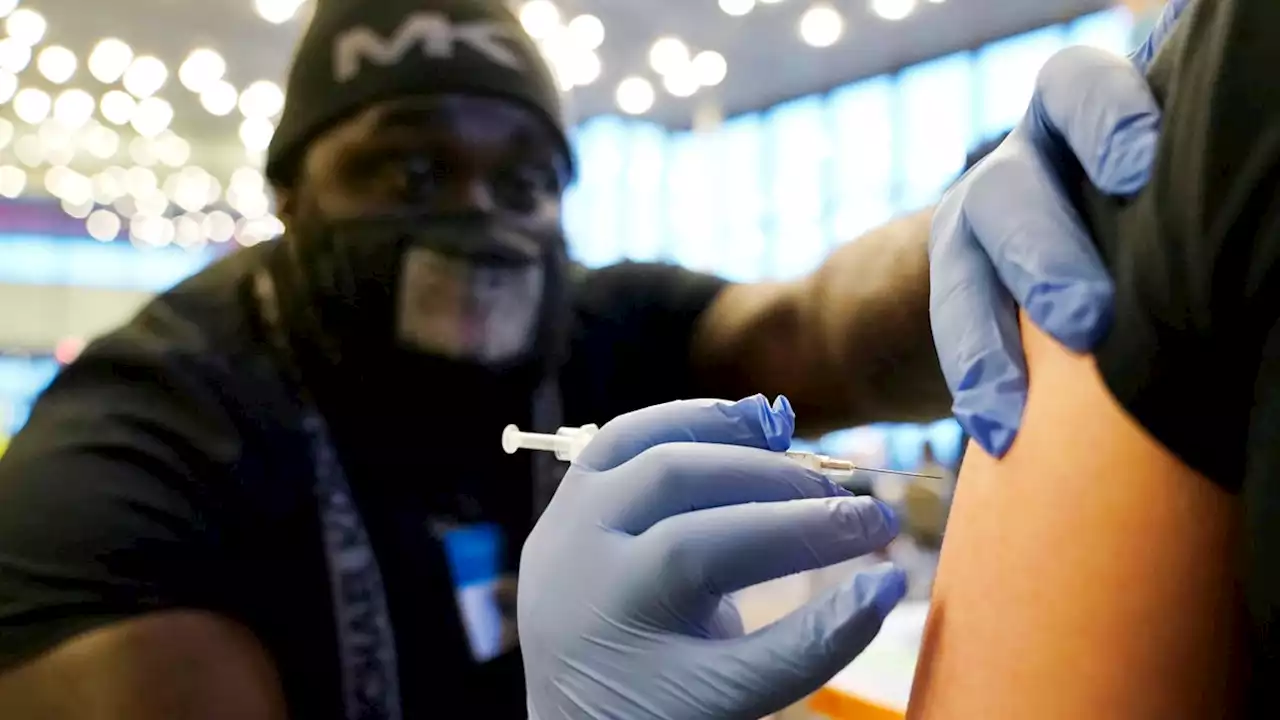
People age 50 and older are eligible for a second booster dose of COVID-19 vaccine at least four months after their first, the Food and Drug Administration said today. The CDC has to sign off on the booster before it becomes available.
COVID case rates are rising in Europe and Asia. Here's what that means for the United States.People age 50 and up are eligible for a second booster dose of COVID-19 vaccine at least four months after their first, the Food and Drug Administration said Tuesday.
The Centers for Disease Control and Prevention has to sign off on the booster before it becomes available. It's not clear how soon that may happen. "Obviously, the older you are, the higher the risk; and the more underlying conditions, the higher the risk," said Dr. Tom Frieden, former director of the CDC, who heads Resolve to Save Lives, an initiative to prevent epidemics and cardiovascular disease.
Anyone 12 or older with a weakened immune system because of medical treatment or conditions is eligible for an additional shot aimed to provide them the same level of protection someone with a healthy immune system received after three doses. Immunocompromised adults can receive a Moderna booster, though it is not authorized for use in minors.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
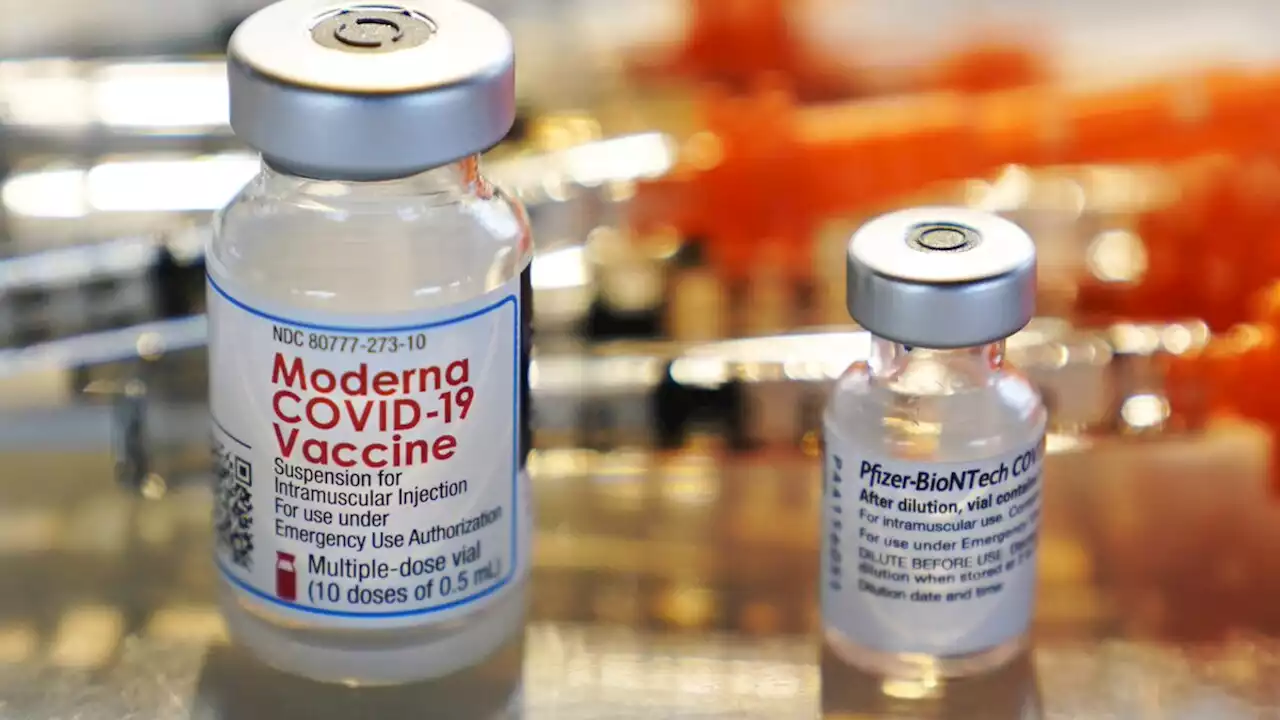 FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
FDA authorizes second COVID-19 booster shot for people 50 and olderAdults 50 and older are now eligible to receive a second COVID-19 booster shot.
Read more »
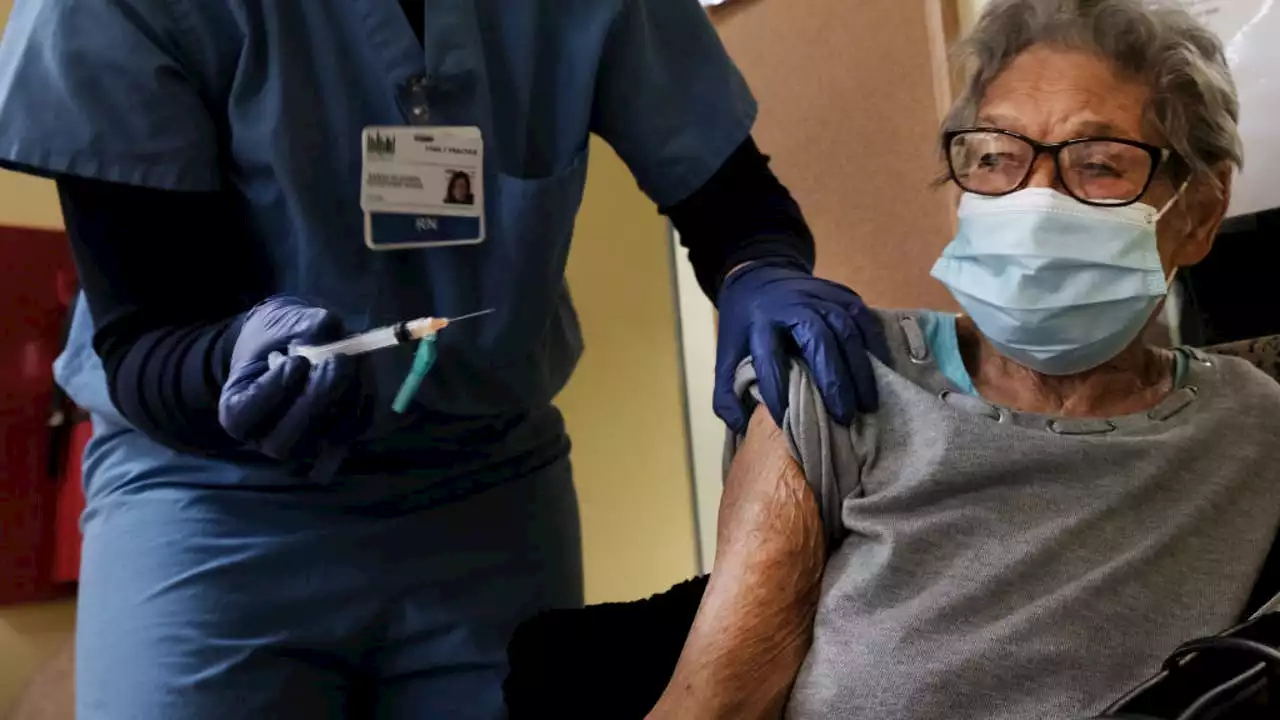 FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA approves 2nd COVID-19 vaccine booster for those 50 and olderU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Read more »
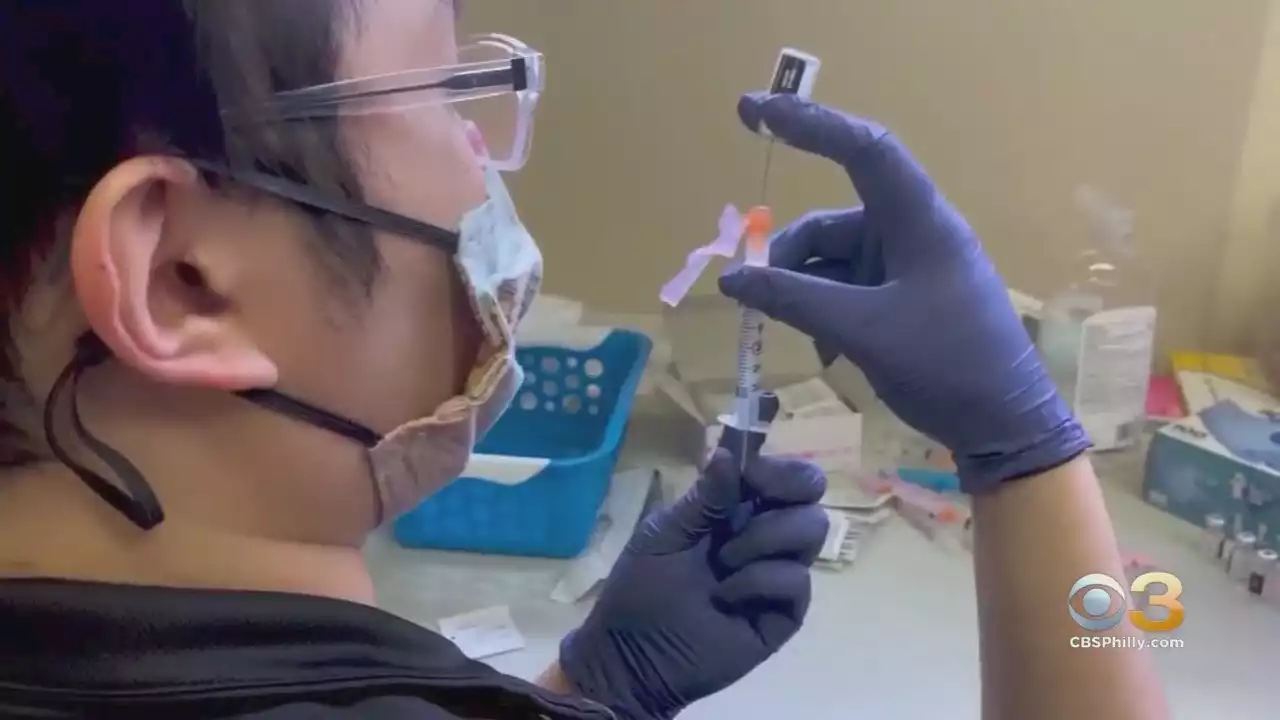 FDA Authorizes Second COVID-19 Booster Shots For Adults Age 50 And OlderJUST IN: The US_FDA has expanded the emergency use authorization of the Pfizer and Moderna COVID-19 vaccines to allow adults age 50 and older to get a second booster as early as 4 months after their first booster dose of any COVID-19 vaccine
FDA Authorizes Second COVID-19 Booster Shots For Adults Age 50 And OlderJUST IN: The US_FDA has expanded the emergency use authorization of the Pfizer and Moderna COVID-19 vaccines to allow adults age 50 and older to get a second booster as early as 4 months after their first booster dose of any COVID-19 vaccine
Read more »