The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
The U.S. on Wednesday authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave.
There's one more step before parents can bring their kids in for the new shot: The Centers for Disease Control and Prevention, which recommends how vaccines are used, must sign off. "We want to have the best of both worlds," Pfizer's Dr. Bill Gruber, a pediatrician, told The Associated Press. He hopes the updated shots will "re-energize interest in protecting children for the winter."
Only people who've gotten their initial vaccinations -- with any of the original-formula versions -- qualify for an updated booster. That means about three-fourths of Americans 12 and older are eligible. As of last weekend, only at least 13 million had gotten an updated booster, White House COVID-19 coordinator Dr. Ashish Jha estimated Tuesday.
Pfizer said it could ship up to 6 million kid-sized doses within a week of authorization, in addition to ongoing adult-dose shipments. Exactly how much protection does an updated COVID-19 booster shot offer? That's hard to know. Pfizer and Moderna are starting studies in young children.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA clears updated COVID boosters for kids as young as 5The FDA has authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave.
FDA clears updated COVID boosters for kids as young as 5The FDA has authorized updated COVID-19 boosters for children as young as 5, seeking to expand protection ahead of an expected winter wave.
Read more »
 FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
Read more »
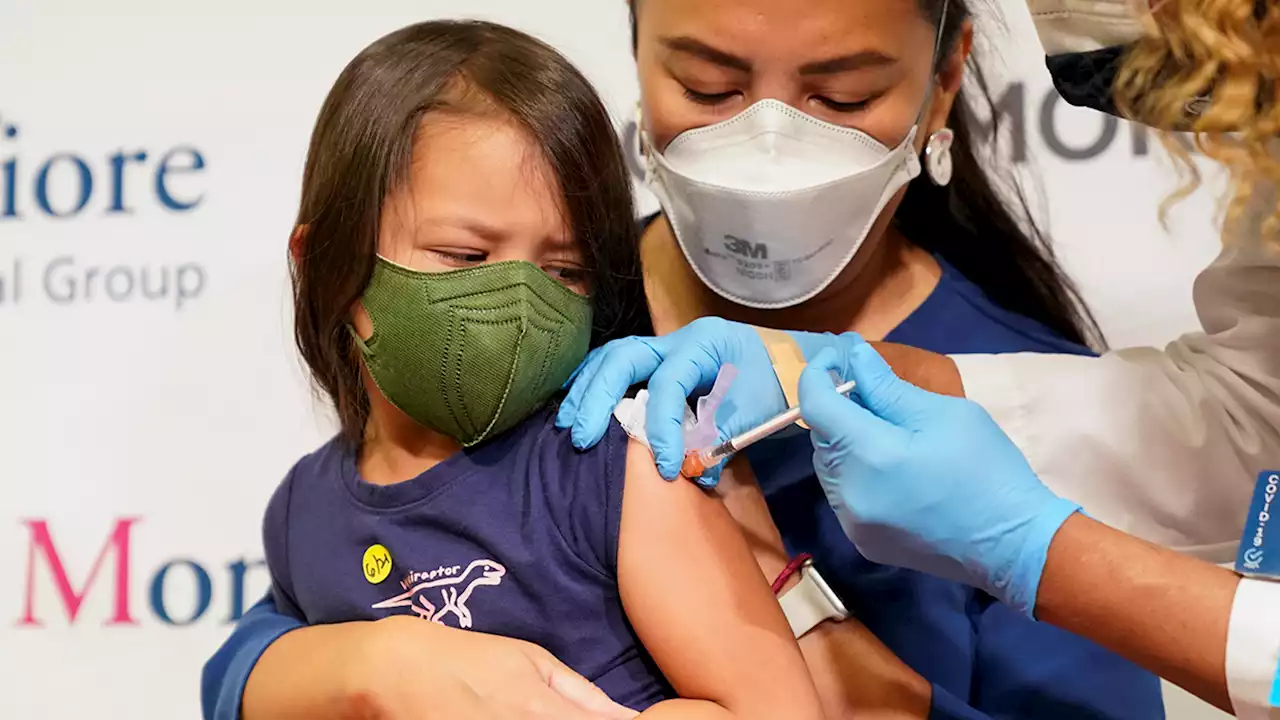 FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
FDA authorizes updated COVID-19 boosters for children as young as 5The updated shots contain half the recipe that targeted the original coronavirus strain and half protection against the dominant BA.4 and BA.5 omicron versions.
Read more »
 FDA authorizes bivalent COVID-19 boosters for children ages 5 to 11Children ages 5 to 11 can now get a COVID booster after the FDA authorized the bivalent dose Wednesday from both Pfizer-BioNTech and Moderna.
FDA authorizes bivalent COVID-19 boosters for children ages 5 to 11Children ages 5 to 11 can now get a COVID booster after the FDA authorized the bivalent dose Wednesday from both Pfizer-BioNTech and Moderna.
Read more »
 FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
FDA authorizes omicron-targeting COVID-19 booster for younger kidsThe Food and Drug Administration authorized the bivalent COVID-19 booster shot for younger children. Moderna's booster is authorized for children six years and older.
Read more »
