BREAKING: The FDA has now authorized bivalent COVID-19 boosters for everyone 6 months and older.
booster, available from both Pfizer and Moderna, was previously authorized"More children now have the opportunity to update their protection against COVID-19 with a bivalent COVID-19 vaccine, and we encourage parents and caregivers of those eligible to consider doing so -- especially as we head into the holidays and winter months where more time will be spent indoors," FDA Commissioner Robert Califf said in a statement.
"As this virus has changed, and immunity from previous COVID-19 vaccination wanes, the more people who keep up to date on COVID-19 vaccinations, the more benefit there will be for individuals, families and public health by helping prevent severe illnesses, hospitalizations, and deaths."Children are eligible to receive the booster at least two months from the completion of their primary series or after receiving a separate booster dose.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
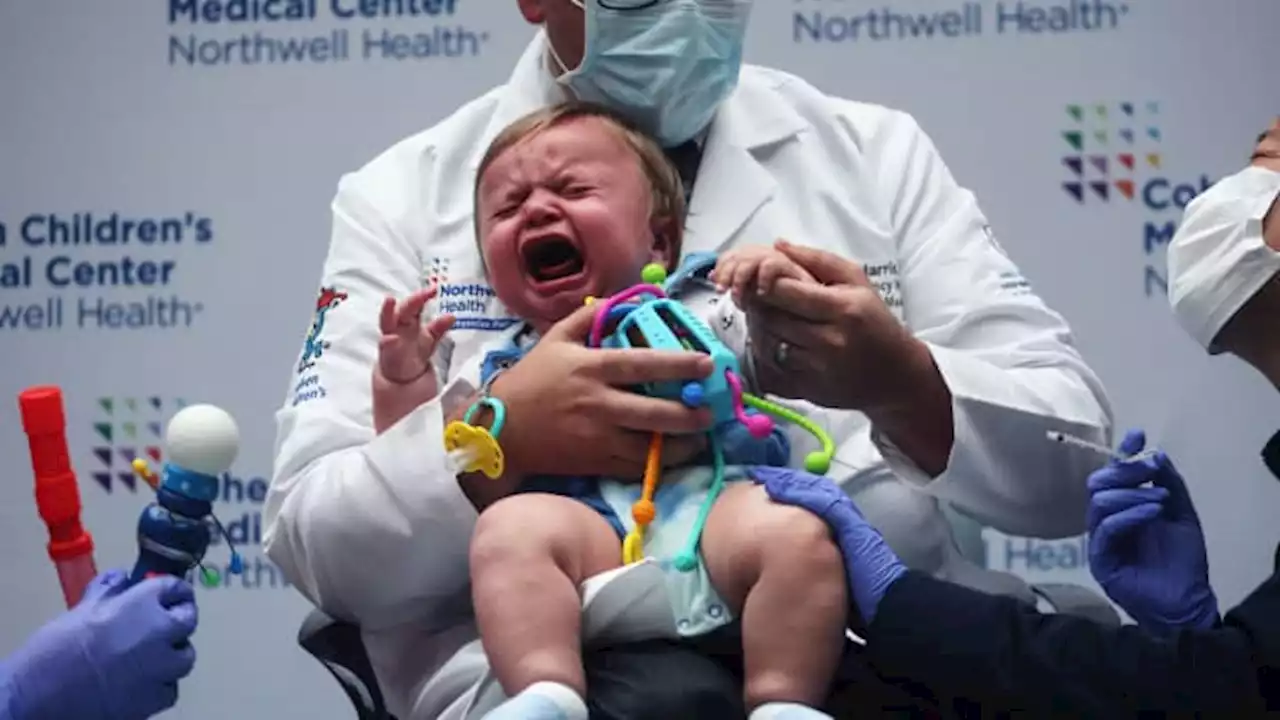 FDA authorizes Covid omicron vaccines for children as young as 6 months old
FDA authorizes Covid omicron vaccines for children as young as 6 months old
Read more »
 Panel calls for stronger leadership of FDA food programsFDA Commissioner Dr. Robert Califf said he would consider which changes to make after reviewing the report and consulting with experts inside and outside the agency.
Panel calls for stronger leadership of FDA food programsFDA Commissioner Dr. Robert Califf said he would consider which changes to make after reviewing the report and consulting with experts inside and outside the agency.
Read more »
 InnovationRx: Healthcare’s Power Women, China Loosens Covid Rules And A Boost For Value-Based CareInnovationRx is your weekly digest of healthcare news. Sign up!
InnovationRx: Healthcare’s Power Women, China Loosens Covid Rules And A Boost For Value-Based CareInnovationRx is your weekly digest of healthcare news. Sign up!
Read more »
 Food For Bay Area Families: Shop local and support local vendors and food banksReed Cowan is at Whole Foods Market in Oakland and tells us how people can shop local and support local vendors and food banks.
Food For Bay Area Families: Shop local and support local vendors and food banksReed Cowan is at Whole Foods Market in Oakland and tells us how people can shop local and support local vendors and food banks.
Read more »
 NJ COVID hospitalizations hit 10-month highThe state is currently seeing the highest number of COVID-19 hospitalizations since February.
NJ COVID hospitalizations hit 10-month highThe state is currently seeing the highest number of COVID-19 hospitalizations since February.
Read more »
