Some Americans will soon be able to get another shot of the bivalent COVID-19 vaccines, the FDA authorized Tuesday, including seniors ages 65 and older and people with compromised immune systems.
Seniors can get the dose as early as four months after their first shot of bivalent vaccine. Immunocompromised Americans can get more doses as early as two months after.
The FDA's new authorizations come as part of a sweeping set of revisions to streamline the myriad of immunization schedules that had been laid out for Moderna and Pfizer-BioNTech, moving them close to the annual seasonal"At this stage of the pandemic, data support simplifying the use of the authorized mRNA bivalent COVID-19 vaccines and the agency believes that this approach will help encourage future vaccination," the FDA's top vaccines official Dr.
"The FDA intends to make decisions about future vaccination after receiving recommendations on the fall strain composition at an FDA advisory committee in June," the regulator said., authorities also will allow still-unvaccinated Americans to bypass the two original monovalent shots that were designed to target the original strain of the virus earlier in the pandemic.
"Evidence is now available that most of the U.S. population 5 years of age and older has antibodies to SARS-CoV-2, the virus that causes COVID-19, either from vaccination or infection that can serve as a foundation for the protection provided by the bivalent vaccines," Marks said.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
FDA okays second omicron booster for people at high risk from covidBreaking news: FDA clears a second omicron-targeting booster shot for people who are at high risk from the coronavirus
Read more »
 FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
FDA authorizes second bivalent COVID booster for older adultsThe U.S. Food and Drug Administration on Tuesday authorized a second dose of Omicron-targeting COVID-19 vaccines for older adults as well as those with a weak immune system.
Read more »
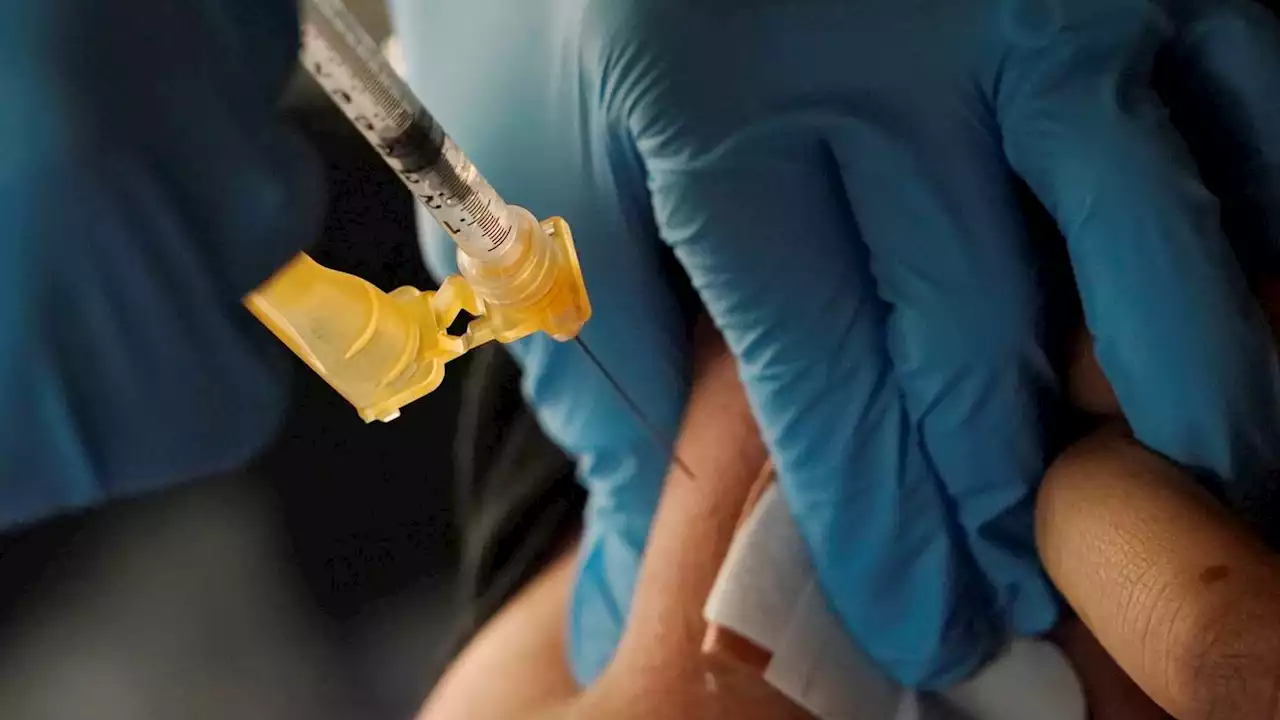 Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring
Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk from COVID-19 can get an extra vaccine booster this spring
Read more »
 Extra spring COVID booster cleared for certain AmericansThe FDA said cleared a second booster for anyone 65 or older as long as it’s been at least four months since their first dose.
Extra spring COVID booster cleared for certain AmericansThe FDA said cleared a second booster for anyone 65 or older as long as it’s been at least four months since their first dose.
Read more »
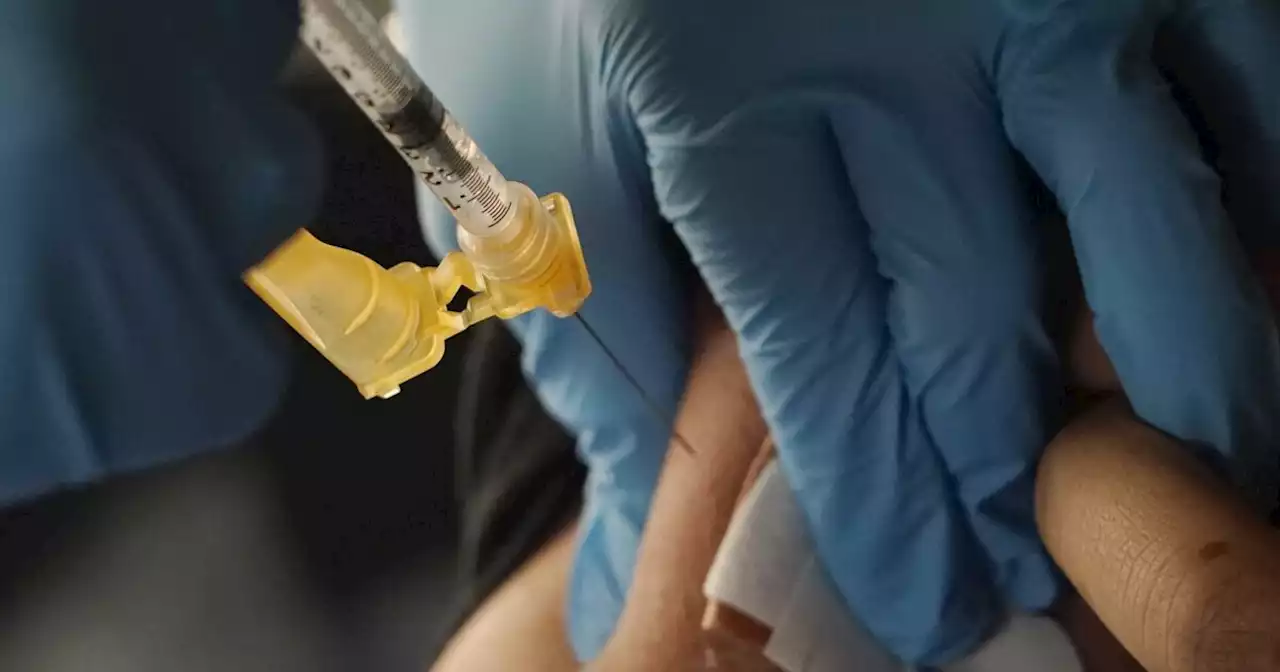 Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk of becoming seriously ill from COVID-19 will be able to get an extra bivalent vaccine booster this spring, the FDA says.
Extra spring COVID booster cleared for certain AmericansCertain Americans at high risk of becoming seriously ill from COVID-19 will be able to get an extra bivalent vaccine booster this spring, the FDA says.
Read more »
 Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Supreme Court to Decide Future of Abortion Pill MifepristoneWATCH: The FDA approved mifepristone initially some 20 years ago, but now the question is whether the FDA did it the right way, says legal analyst Dan Eaton.
Read more »
