A key FDA advisory committee voted unanimously to recommend Moderna's COVID vaccine receive emergency use authorization for kids as young as 6 months old. They have another vote scheduled today for the Pfizer-BioNTech vaccine.
This past weekend, the FDA released its analysis showing that shots from both companies were "safe and effective."If FDA commissioner Robert Califf follows their recommendation and ultimately approves the authorization as expected, the CDC will then weigh whether to issue a recommendation of Moderna's shots in this age group.
If all goes according to plan, shots could become available as soon as June 21 since many doctor's offices may be closed for the federal Juneteenth holiday on June 20,On Wednesday, the FDA committee was considering whether the benefits of using either the Moderna and Pfizer shots in this age group outweighed the risks.
The committee also voted in favor of the two-dose series of the Moderna vaccine for use in infants and children 6 months through 5 years of age.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
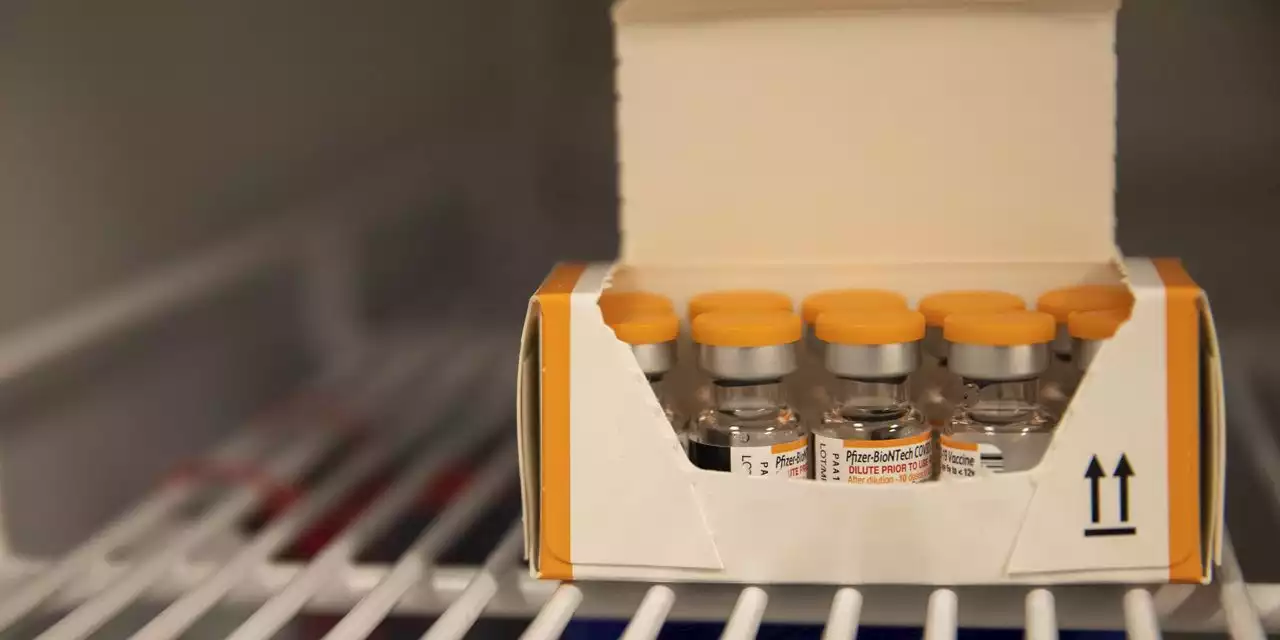 FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
FDA Advisers Review Pfizer, Moderna Covid-19 Vaccines in Young ChildrenThe advisory panel’s meeting moves the Pfizer and Moderna shots one step closer to authorization for one of the last groups of people in the U.S. who haven’t been able to get vaccinated against the coronavirus.
Read more »
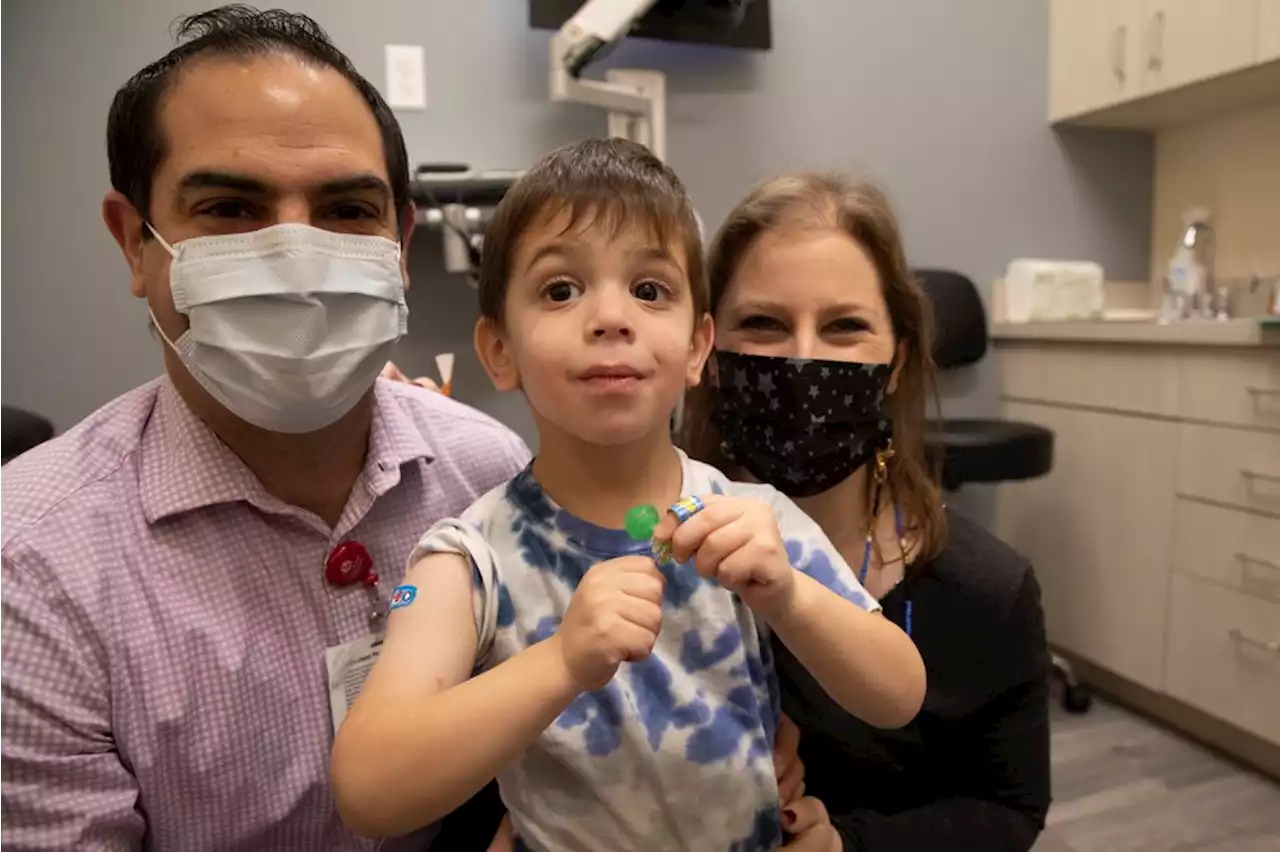
 FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
FDA Advisers Review Moderna, Pfizer COVID-19 Shots for Kids as Young as 6 monthsU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for babies, toddlers and preschoolers, moving the nation closer to vaccinations for all ages.
Read more »
 FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
FDA advisers weigh COVID-19 shots for babies, young childrenU.S. government advisers met Wednesday to decide whether to endorse COVID-19 shots for the youngest children, moving the nation closer to vaccinations for all ages.
Read more »
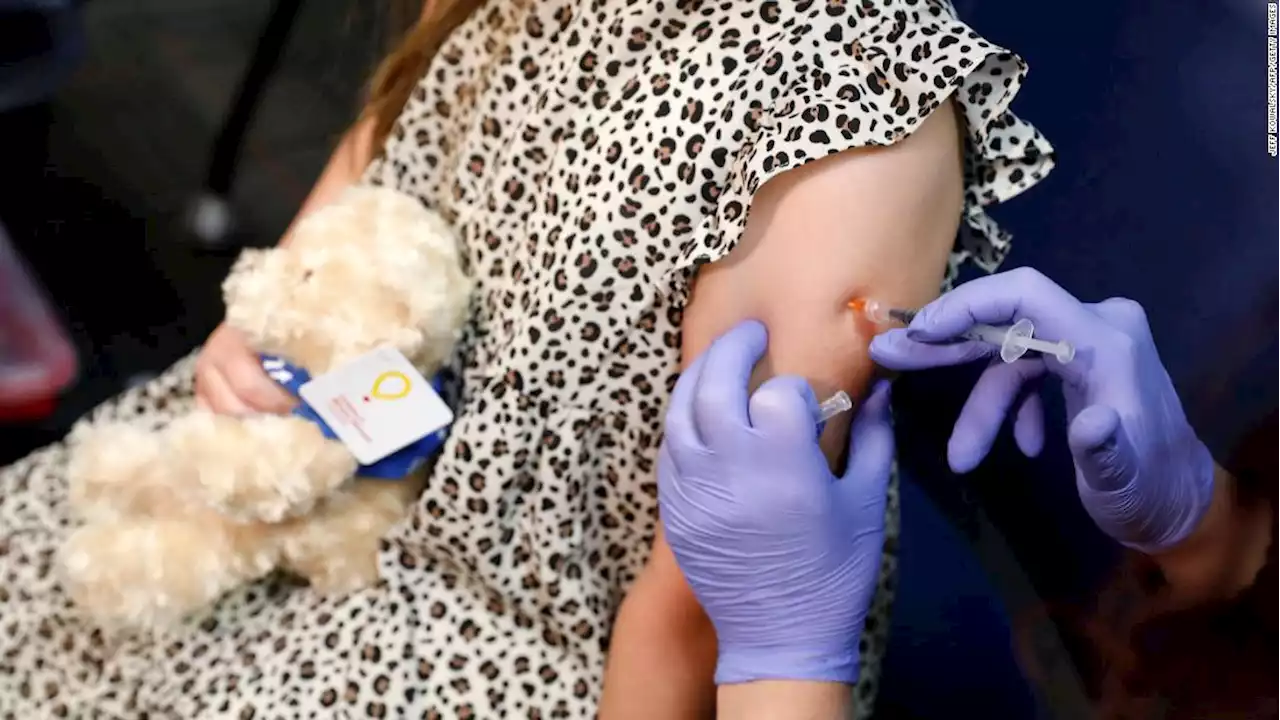 FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
FDA advisers to weigh expanding Covid-19 vaccines to younger childrenSeveral months after older children became eligible to get vaccinated against Covid-19, the United States might be just days away from offering vaccines to those younger than 5.
Read more »
 FDA Recommends the Emergency Authorization of Moderna's COVID-19 Vaccine for Children Ages 6 to 17An expert panel recommended that kids have another option when it comes to getting vaccinated against COVID-19
FDA Recommends the Emergency Authorization of Moderna's COVID-19 Vaccine for Children Ages 6 to 17An expert panel recommended that kids have another option when it comes to getting vaccinated against COVID-19
Read more »
