Do I have Covid or the flu?: FDA greenlights nonprescription, at-home test that can detect Covid, influenza A and B, and RSV.
The test, manufactured by North Carolina-based company Labcorp, allows people to collect their own nasal swab sample at home and then ship it to one of Labcorp's labs to be analyzed.
Labcorp's newly authorized test removes some of the red tape, allowing Americans to collect a sample from the convenience of their own home. “Technology is advancing very quickly, and in due time, home-based point of care testing using PCR will likely be an option in the near future,” she said.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
FDA authorizes first non-prescription test for COVID-19, flu, RSVNEW: The FDA has authorized the first non-prescription COVID-19 test that can also detect the flu and RSV.
Read more »
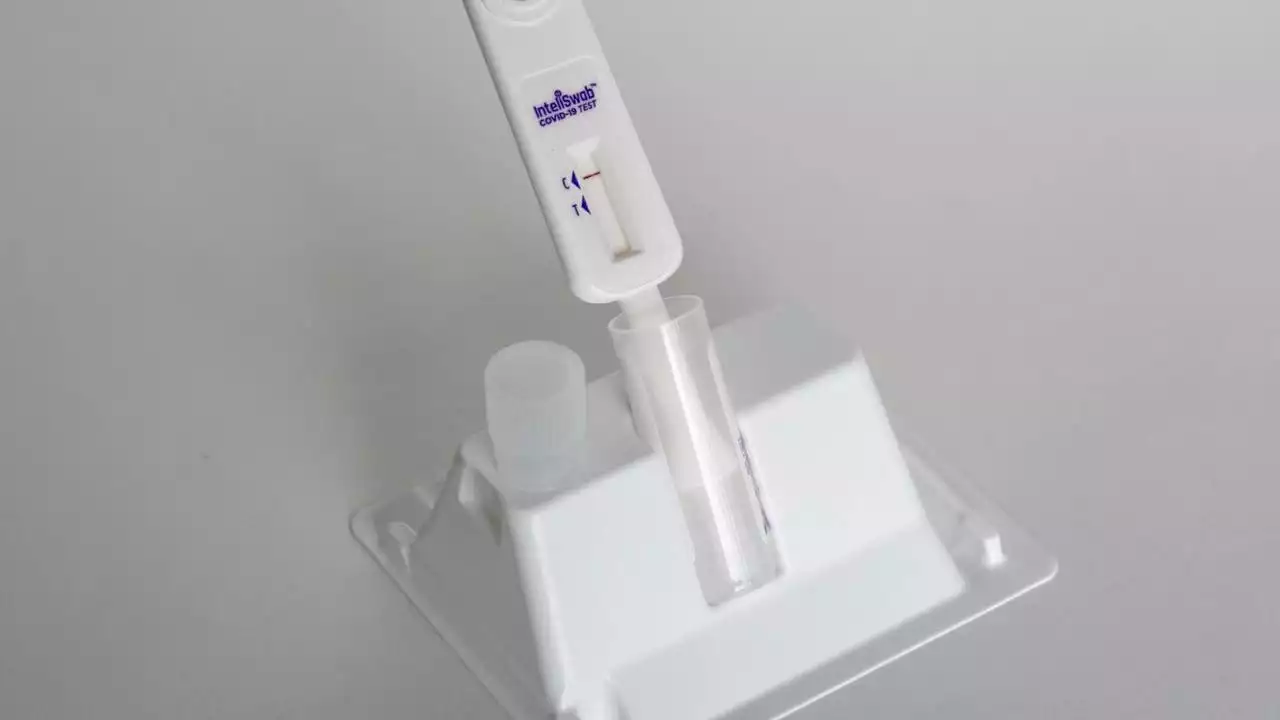 FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
FDA approves first non-prescription COVID test that also detects flu, RSVThe U.S. Food and Drug Administration issued its approval Monday of the first non-prescription at-home COVID-19 test that also detects the flu.
Read more »
 FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
FDA Authorizes Nonprescription Test for Covid-19, Flu and RSVThe FDA authorized the first nonprescription test for Covid-19, influenza and RSV, in another step toward increasing patient access to tests for Covid-19 and other conditions
Read more »
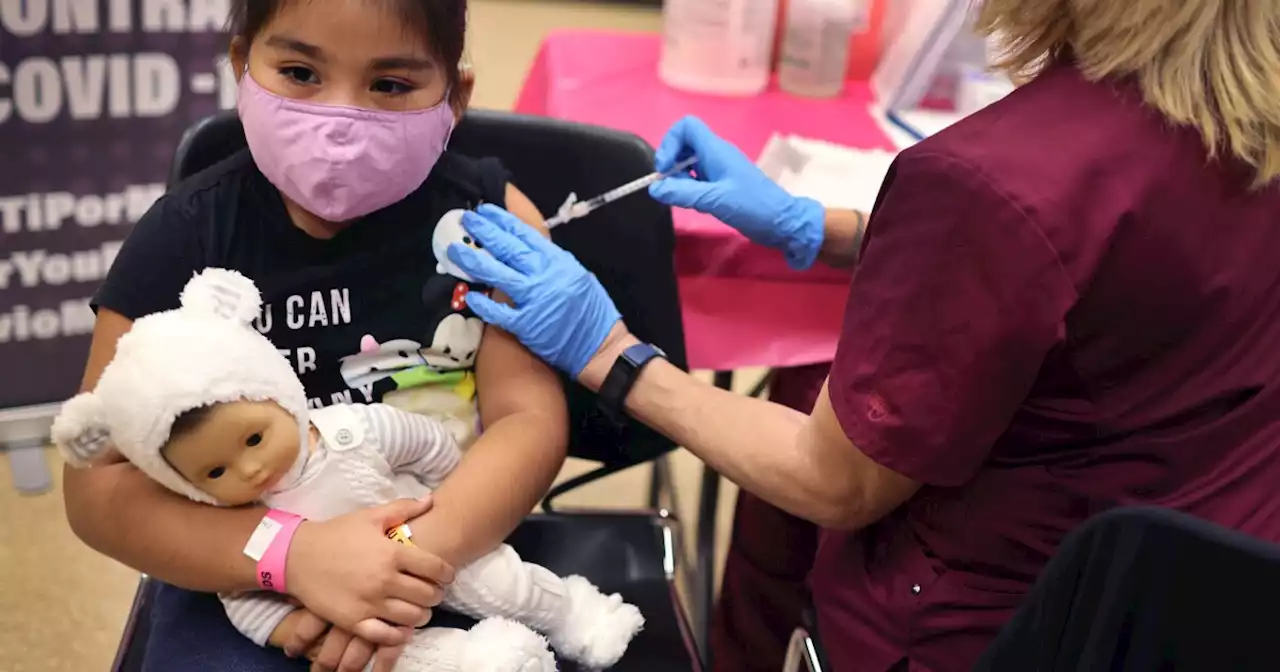 FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
FDA authorizes first COVID booster for children ages 5 to 11The Food and Drug Administration expanded authorization of Pfizer-BioNTech's COVID vaccine to enable kids ages 5 to 11 who were vaccinated at least five months ago to get a third shot.
Read more »
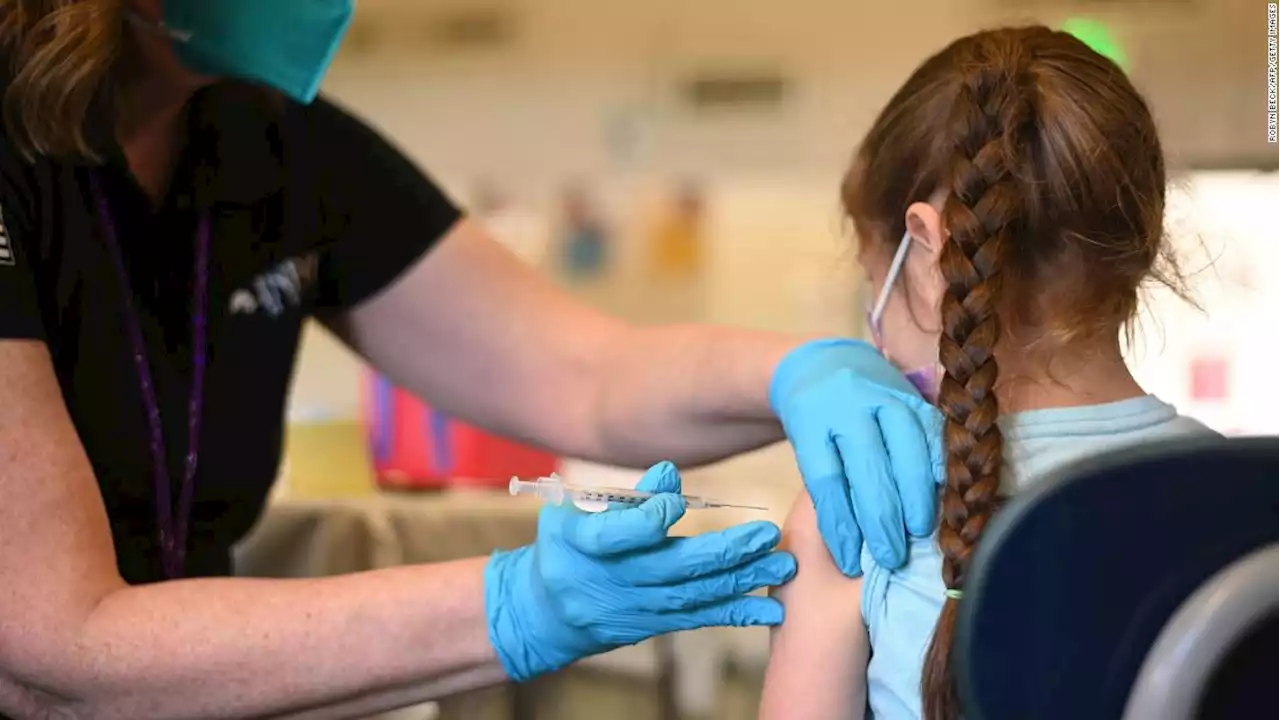 FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
FDA authorizes Pfizer Covid-19 booster shots for children ages 5 to 11The US Food and Drug Administration has granted emergency use authorization for a booster dose of Pfizer/BioNTech's Covid-19 vaccine for children ages 5 to 11 at least five months after completion of the primary vaccine series.
Read more »
 FDA Clears Pfizer’s COVID Boosters for Ages 5–11 — But What’s the Timeline for Younger Kids?Here's what parents need to know about Pfizer's COVID19 boosters for kids this spring and summer.
FDA Clears Pfizer’s COVID Boosters for Ages 5–11 — But What’s the Timeline for Younger Kids?Here's what parents need to know about Pfizer's COVID19 boosters for kids this spring and summer.
Read more »
