The U.S. Food and Drug Administration has authorized another booster dose of the Pfizer or Moderna COVID-19 vaccine for people ages 50 and up.
— even as vaccination has stalled. About two-thirds of Americans are fully vaccinated, and half of those eligible for a first booster haven’t gotten one.
There’s limited evidence to tell how much benefit another booster could offer right now. FDA made the decision without input from its independent panel of experts that has “There might be a reason to top off the tanks a little bit” for older people and those with other health conditions, said University of Pennsylvania immunologist E. John Wherry, who wasn’t involved in the government’s decision.
U.S. health officials also looked to Israel, which during the omicron surge opened a fourth dose to people 60 and older at least four months after their last shot. The FDA said no new safety concerns emerged in a review of 700,000 fourth doses administered. “The ‘when’ is a really difficult part. Ideally we would time booster doses right before surges but we don’t always know when that’s going to be,” said Dr. William Moss, a vaccine expert at the Johns Hopkins Bloomberg School of Public Health.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds. The Food and Drug Administration's decision opens a fourth dose of the Pfizer or Moderna vaccines to that age group at least four months after their previous booster. Until now, the FDA had cleared fourth doses only for people 12 and older who have severely weakened immune systems.
FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds. The Food and Drug Administration's decision opens a fourth dose of the Pfizer or Moderna vaccines to that age group at least four months after their previous booster. Until now, the FDA had cleared fourth doses only for people 12 and older who have severely weakened immune systems.
Read more »
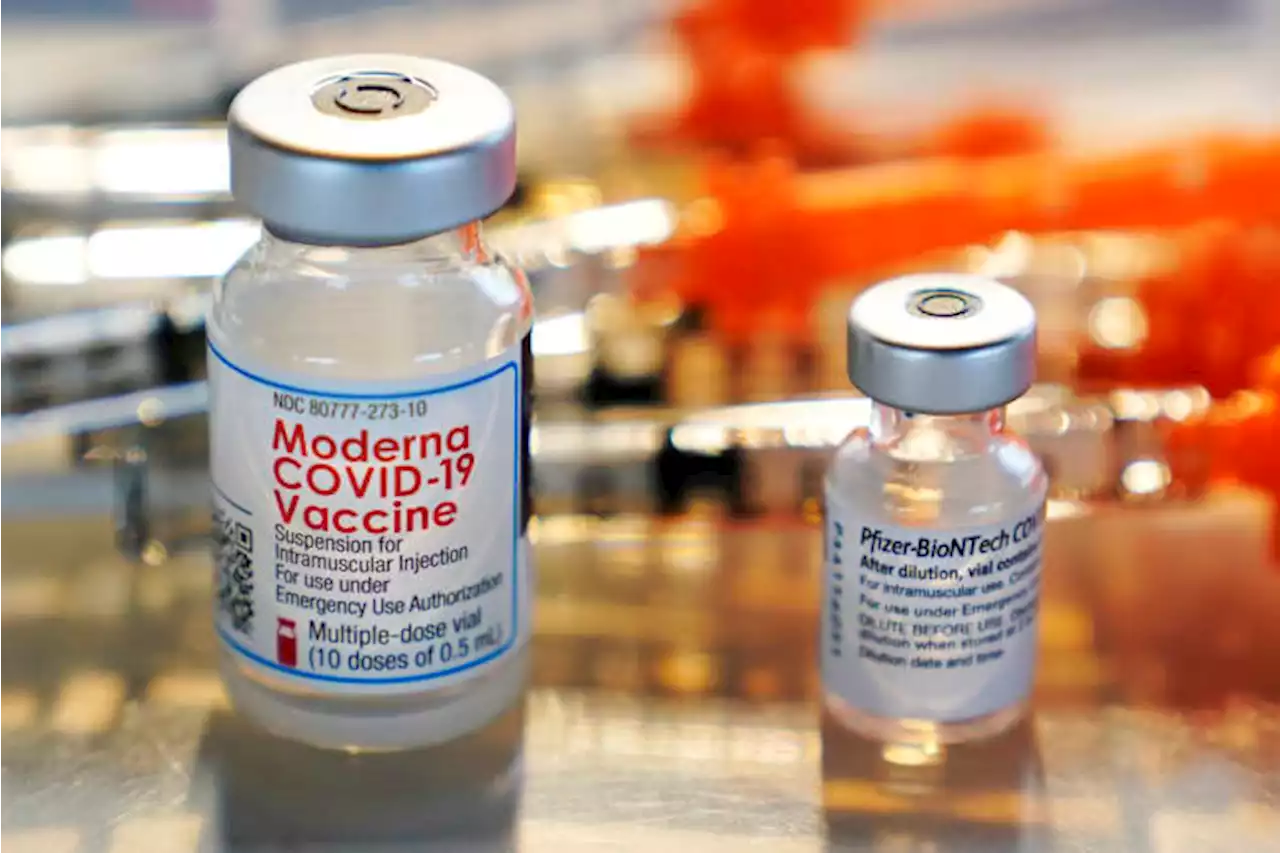 FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Read more »
 FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
FDA OKs another Pfizer, Moderna COVID booster for 50 and upU.S. regulators are allowing people 50 and older to get another booster dose of the Pfizer or Moderna COVID-19 vaccine.
Read more »
FDA OKs second Pfizer, Moderna COVID-19 booster for ages 50 and upU.S. regulators on Tuesday authorized another COVID-19 booster for people age 50 and older, a step to offer extra protection for the most vulnerable in case the coronavirus rebounds.
Read more »
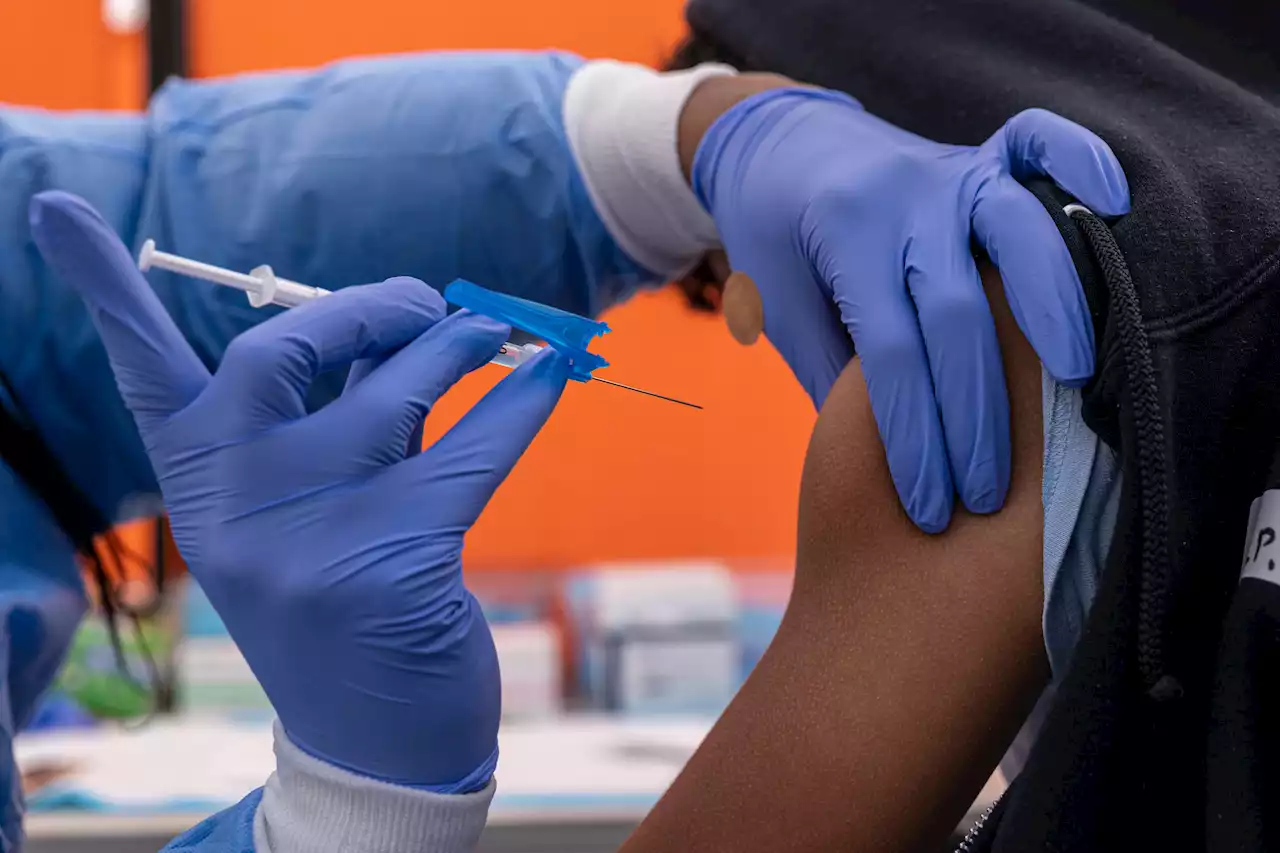 FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
FDA Authorizes Fourth Pfizer and Moderna Covid Vaccine Doses for People Age 50 and OlderThe FDA made the decision comes without consulting its committee of independent vaccine experts.
Read more »
