FDA advisory committee determines phase 3 study of lecanemab, a monoclonal antibody directed against amyloid beta confirms the drug's clinical benefit for patients with Alzheimer's disease.
Members of a US Food and Drug Administration advisory committee have unanimously concluded that a postmarketing study confirms the benefit of the Alzheimer's drug lecanemab , paving the way for traditional approval.
Patients were randomly assigned to receive placebo or lecanemab 10 mg/kg biweekly. In addition to a placebo-controlled period and safety follow-up, the study has an ongoing extension phase of up to 4 years. The drug also affected function, with a 37% decrease compared to placebo on the AD Cooperative Study–Activities of Daily Living Scale for Mild Cognitive Impairment.
Lecanemab also affected biomarkers of amyloid, tau, and neurodegeneration, providing a biological basis for the treatment effects consistent with slowing of disease progression.All six committee members agreed by vote that the study provides evidence of clinical benefit. They variously descried the study and results as"robust,""compelling,""well conducted,""clear and consistent," and"clinically meaningful.
"And for serious ARIA, there was an imbalance favoring placebo, but overall, these were pretty rare," he said.Committee members discussed the risk/benefit profile for three subgroups of patients ― those with apolipoprotein E 4 allele, patients taking an anticoagulant, and those with cerebral amyloid angiopathy .
With respect to CAA, most members supported the idea of considering use of the drug in the presence of this condition, but only after discussing the risks with patients and their families and in the presence of a robust reporting system.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Alzheimer's drug gets FDA panel's backing, setting the stage for broader useHealth advisers on Friday unanimously backed the full approval of a closely watched Alzheimer's drug, a key step toward opening insurance coverage to U.S. seniors with early stages of the brain-robbing disease.
Alzheimer's drug gets FDA panel's backing, setting the stage for broader useHealth advisers on Friday unanimously backed the full approval of a closely watched Alzheimer's drug, a key step toward opening insurance coverage to U.S. seniors with early stages of the brain-robbing disease.
Read more »
 FDA paves way for full approval of new Alzheimer's drugA panel of Food and Drug Administration advisers on Friday unanimously endorsed an experimental Alzheimer's drug that's been shown to have modest success slowing the progression of the disease, clearing a hurdle for full agency approval.
FDA paves way for full approval of new Alzheimer's drugA panel of Food and Drug Administration advisers on Friday unanimously endorsed an experimental Alzheimer's drug that's been shown to have modest success slowing the progression of the disease, clearing a hurdle for full agency approval.
Read more »
 Biden's FDA clears path for Chinese products to flood US tobacco, nicotine marketThe FDA is under fire for allegedly caving to political pressure to ban vapes and e-cigarettes while simultaneously allowing Chinese products to flood the U.S. market.
Biden's FDA clears path for Chinese products to flood US tobacco, nicotine marketThe FDA is under fire for allegedly caving to political pressure to ban vapes and e-cigarettes while simultaneously allowing Chinese products to flood the U.S. market.
Read more »
 Dozens turn out for Strolling for Alzheimer’s Caregivers WalkathonThe Doris Marie Jones Foundation held the event at Highpoint Park to help raise awareness for the caregivers of the those afflicted with the disease.
Dozens turn out for Strolling for Alzheimer’s Caregivers WalkathonThe Doris Marie Jones Foundation held the event at Highpoint Park to help raise awareness for the caregivers of the those afflicted with the disease.
Read more »
 FDA Flags Getinge/Maquet Recall of Oxygenator DevicesGetinge/Maquet recalls all Quadrox oxygenators and certain venous hardshell cardiotomy reservoirs due to a potential sterility issue with packaging that could lead to risk for infection and patient harm.
FDA Flags Getinge/Maquet Recall of Oxygenator DevicesGetinge/Maquet recalls all Quadrox oxygenators and certain venous hardshell cardiotomy reservoirs due to a potential sterility issue with packaging that could lead to risk for infection and patient harm.
Read more »
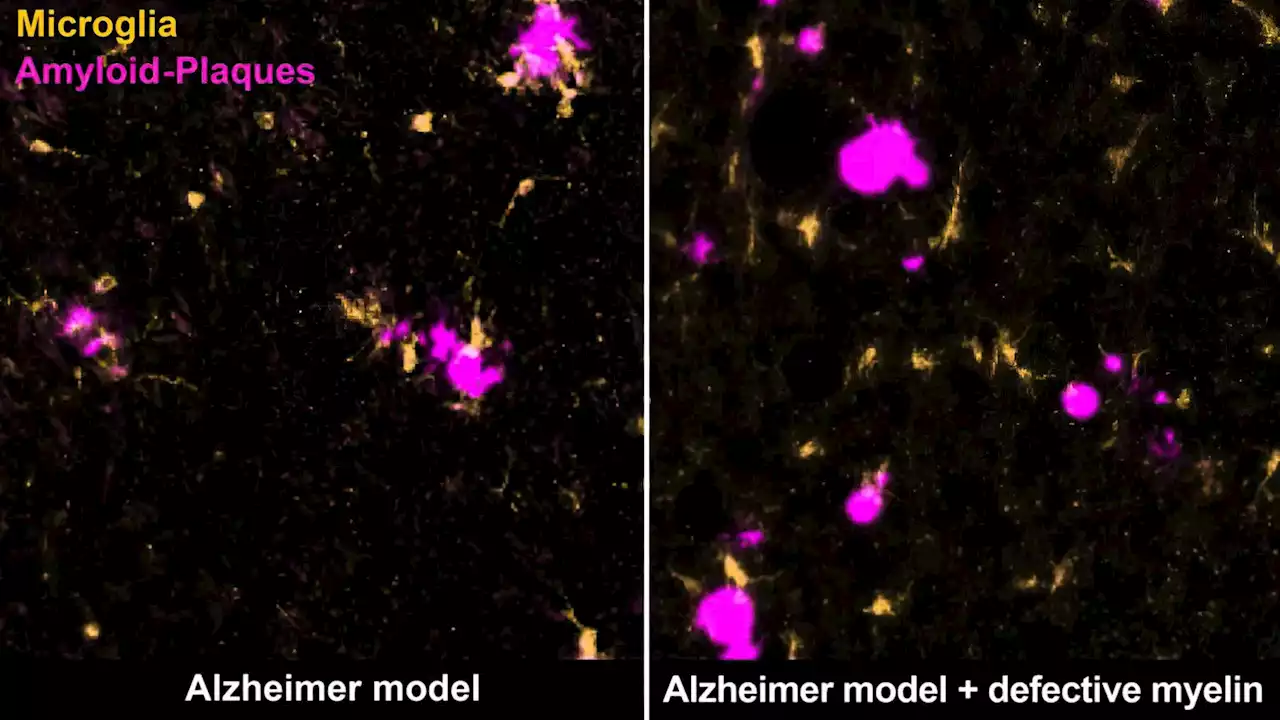 New Hope in Alzheimer’s Fight: Addressing Myelin Degradation Could Prevent DiseaseNew research has demonstrated that impaired myelin actively promotes disease-related changes in Alzheimer’s disease. Alzheimer's disease, an irreversible e type of dementia, is the most prevalent neurodegenerative disease globally. Age is the predominant risk factor for this disease, but the reason
New Hope in Alzheimer’s Fight: Addressing Myelin Degradation Could Prevent DiseaseNew research has demonstrated that impaired myelin actively promotes disease-related changes in Alzheimer’s disease. Alzheimer's disease, an irreversible e type of dementia, is the most prevalent neurodegenerative disease globally. Age is the predominant risk factor for this disease, but the reason
Read more »
