France has entered the first phase of easing Covid-19 restrictions, as the country witnesses a stabilization of the Omicron wave.
A street scene in Paris last week. The outdoor mask mandate is set to end Wednesday and working from home will no longer be mandatory but recommended.
The outdoor mask mandate also ends on Wednesday and working from home will no longer be mandatory but recommended. The relaxation of Covid-19 measures comes as France is still witnessing a high number of daily Covid-19 infections, about 322,000 cases on average a day in the past week, but with a stabilization of intensive care unit cases.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer Asks FDA to Allow COVID-19 Vaccine for Kids Under 5The nation’s 19 million children under 5 are the only group not yet eligible for vaccination against the coronavirus.
Pfizer Asks FDA to Allow COVID-19 Vaccine for Kids Under 5The nation’s 19 million children under 5 are the only group not yet eligible for vaccination against the coronavirus.
Read more »
 Pfizer asks FDA to allow COVID-19 vaccine for kids under 5The move could open the way for the very youngest in the country to start receiving shots as early as March.
Pfizer asks FDA to allow COVID-19 vaccine for kids under 5The move could open the way for the very youngest in the country to start receiving shots as early as March.
Read more »
 Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Regulators urge Pfizer to apply for COVID-19 vaccines for kids under 5Early Pfizer data has shown the vaccine — which is administered to younger kids at one-tenth the strength of the adult shot — is safe and produces an immune response.
Read more »
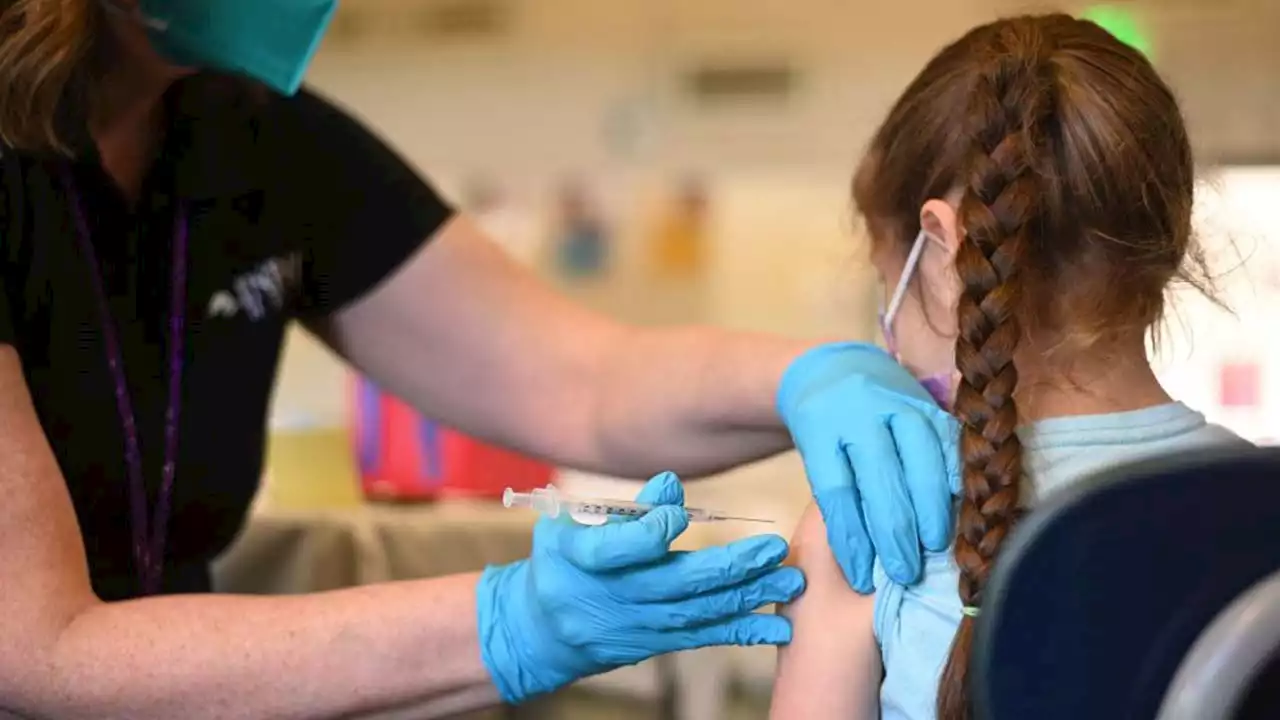 Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Pfizer asks FDA for emergency approval of COVID-19 vaccine for kids under 5VACCINE FOR KIDS: Children 6 months to 5 years old are one step closer to receiving the Pfizer COVID-19 vaccine after the company officially submitted the two-dose regimen to the FDA for emergency approval.
Read more »
 Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Pfizer submits request for COVID-19 vaccine use in children under age 5Pfizer and BioNTech said Tuesday that the companies had asked the Food and Drug Administration (FDA) to authorize their COVID-19 vaccine for emergency use in children younger than 5 years old.
Read more »
 Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Pfizer asks FDA to allow COVID-19 vaccine for kids under 5Pfizer on Tuesday asked the U.S. to authorize extra-low doses of its COVID-19 vaccine for children under 5, potentially opening the way for the very youngest Americans to start receiving shots as early as March.
Read more »
