Gay and bisexual men in monogamous relationships can give blood in the U.S. without abstaining from sex under updated federal health guidelines that focus on donors’ behavior, not their sexual orientation.
WASHINGTON —
Instead, all potential donors — regardless of sexual orientation, sex or gender — will be screened with a new questionnaire that evaluates their individual risks for HIV based on sexual behavior, recent partners and other factors. Potential donors who report having anal sex with new partners in the last three months will be barred from giving until a later date.
Anyone who has ever tested positive for HIV will continue to be ineligible to donate blood. Those taking pills to prevent HIV through sexual contact will also still be barred, until three months after their last dose. The FDA noted that the medications, known as PrEP, can delay the detection of the virus in screening tests.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 UCSF's new drug-gene test could determine if medication is right for youThe way patients are prescribed medicine could soon be a lot more precise. A new form of testing by UCSF could help determine how a patient's body will respond to specific medications and dosages.
UCSF's new drug-gene test could determine if medication is right for youThe way patients are prescribed medicine could soon be a lot more precise. A new form of testing by UCSF could help determine how a patient's body will respond to specific medications and dosages.
Read more »
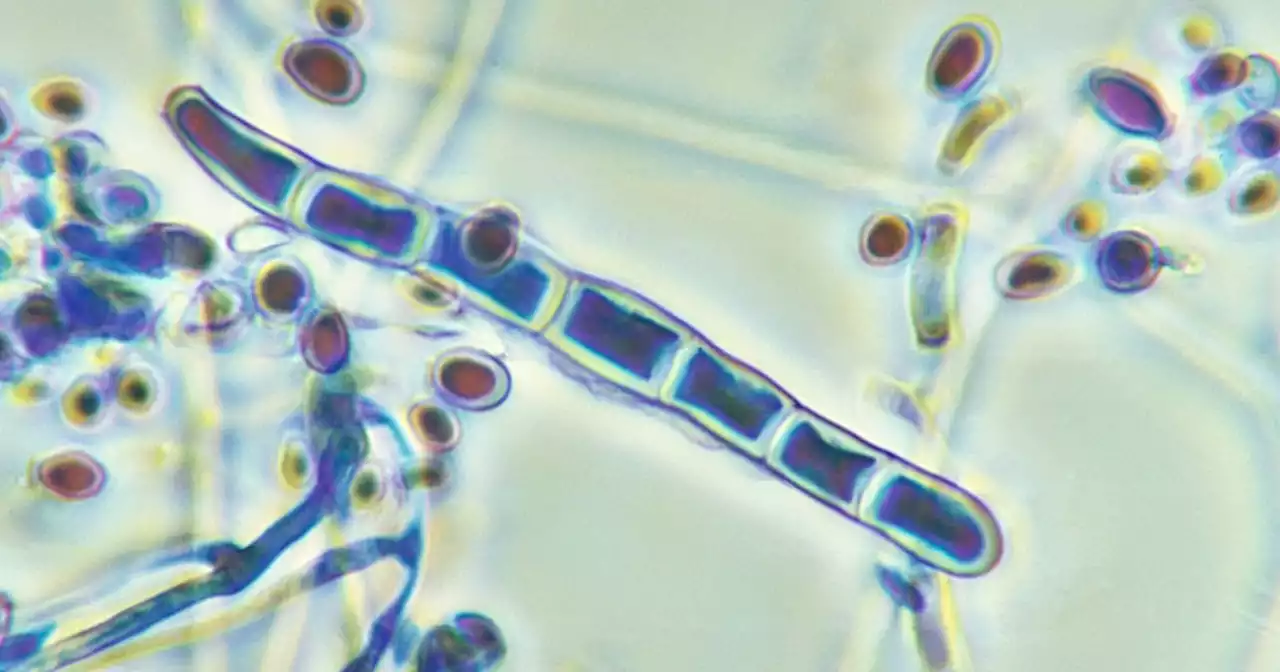 2 cases of drug-resistant ringworm detected in New York City, CDC report findsHere's what we know so far about the cases of a drug-resistant ringworm detected in NYC.
2 cases of drug-resistant ringworm detected in New York City, CDC report findsHere's what we know so far about the cases of a drug-resistant ringworm detected in NYC.
Read more »
 FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
FDA OKs New Drug for Fabry DiseasePegunigalsidase alfa (Elfabrio) is an intravenously administered enzyme replacement therapy to treat adults with confirmed Fabry disease.
Read more »
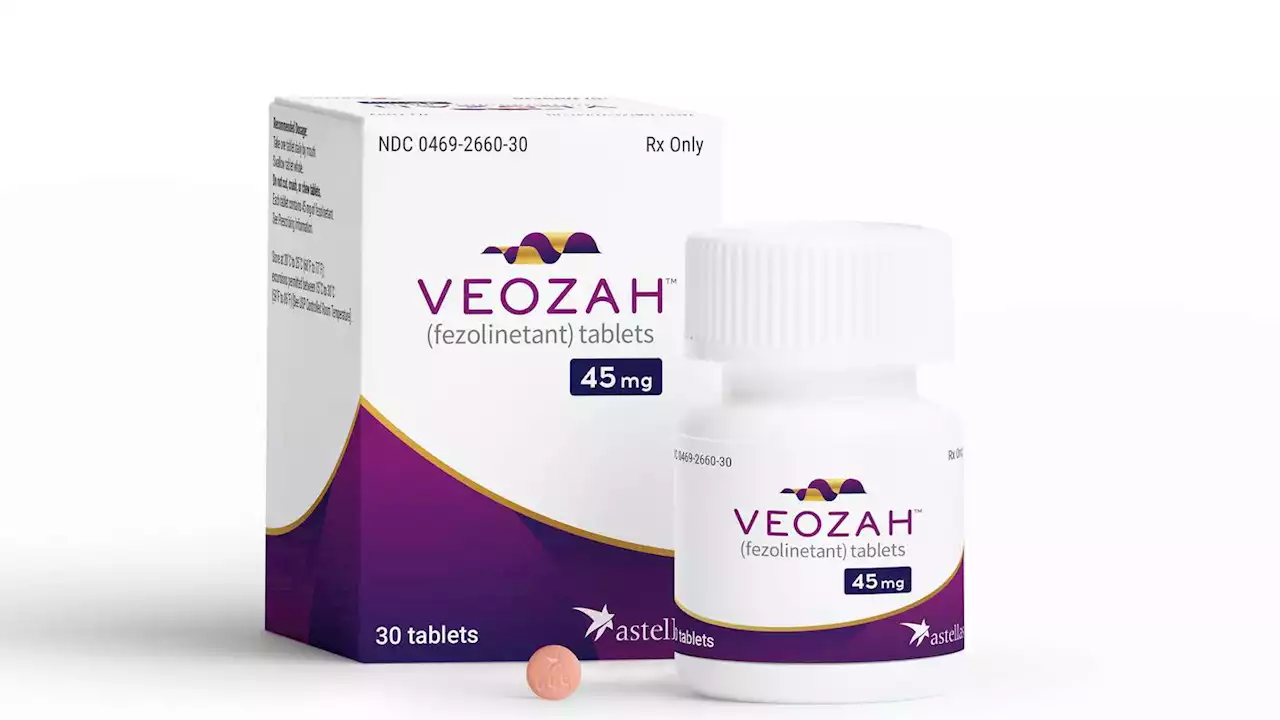 New menopause drug for hot flashes gets FDA approvalThe Food and Drug Administration has approved a new type of drug to treat hot flashes caused by menopause
New menopause drug for hot flashes gets FDA approvalThe Food and Drug Administration has approved a new type of drug to treat hot flashes caused by menopause
Read more »
 New menopause drug for hot flashes gets FDA approvalU.S. health regulators on Friday approved a new type of drug for women dealing with uncomfortable hot flashes caused by menopause. The Food and Drug Administration approved the once-a-day pill from Astellas Pharma to treat moderate-to-severe symptoms, which can include sweating, flushing and chills. Astellas' drug, Veozah, uses a new approach, targeting brain connections that help control body temperature.
New menopause drug for hot flashes gets FDA approvalU.S. health regulators on Friday approved a new type of drug for women dealing with uncomfortable hot flashes caused by menopause. The Food and Drug Administration approved the once-a-day pill from Astellas Pharma to treat moderate-to-severe symptoms, which can include sweating, flushing and chills. Astellas' drug, Veozah, uses a new approach, targeting brain connections that help control body temperature.
Read more »
 FDA OKs New Drug for Hot FlashesFDAapproves fezolinetant, an oral medication for the treatment of moderate to severe hotflashes in menopausal women. menopause womenshealth
FDA OKs New Drug for Hot FlashesFDAapproves fezolinetant, an oral medication for the treatment of moderate to severe hotflashes in menopausal women. menopause womenshealth
Read more »
