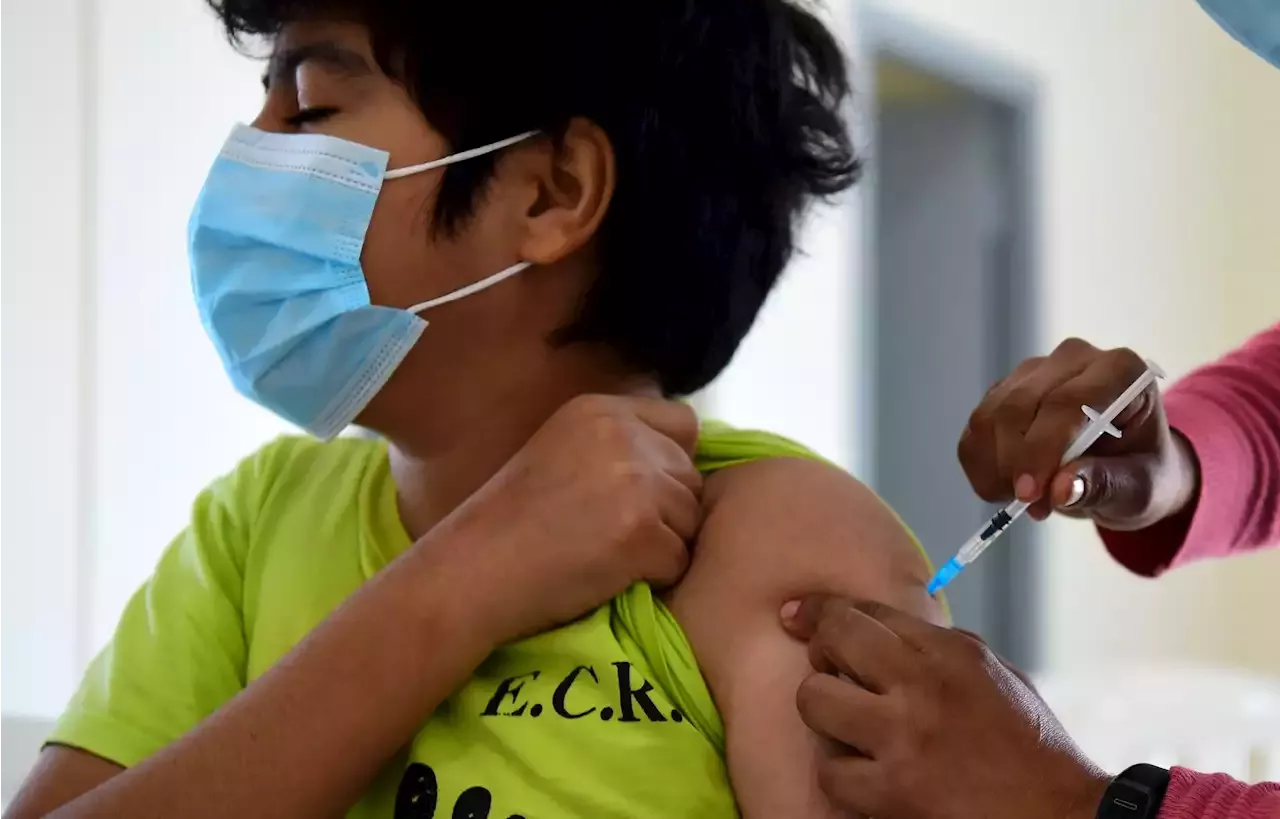
Pfizer and BioNTech requested emergency use authorization from the Food and Drug Administration for a booster shot of their Covid-19 vaccine for kids aged 5-11.
A child receives a dose of the Pfizer-BioNTech's Covid-19 vaccine.indicating that the booster shot created a strong immune response in kids without introducing new safety risks.
Pfizer and BioNTech said they plan to submit data to the European Medicines Agency and to other international regulatory agencies during the next few weeks.The effectiveness of Pfizer’s two-dose vaccine course at preventing Covid-19 infection waned rapidly for children in New York during the Omicron surge, especially those in the 5-11 age bracket, according to
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
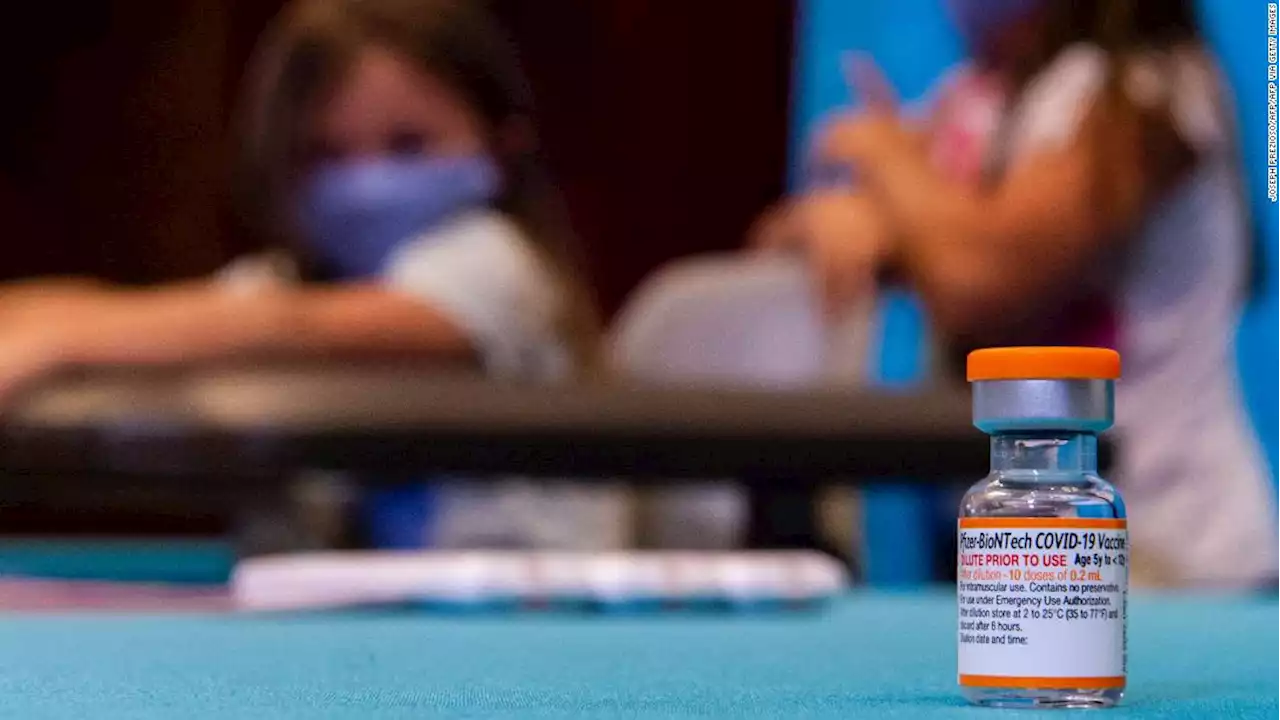 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Read more »
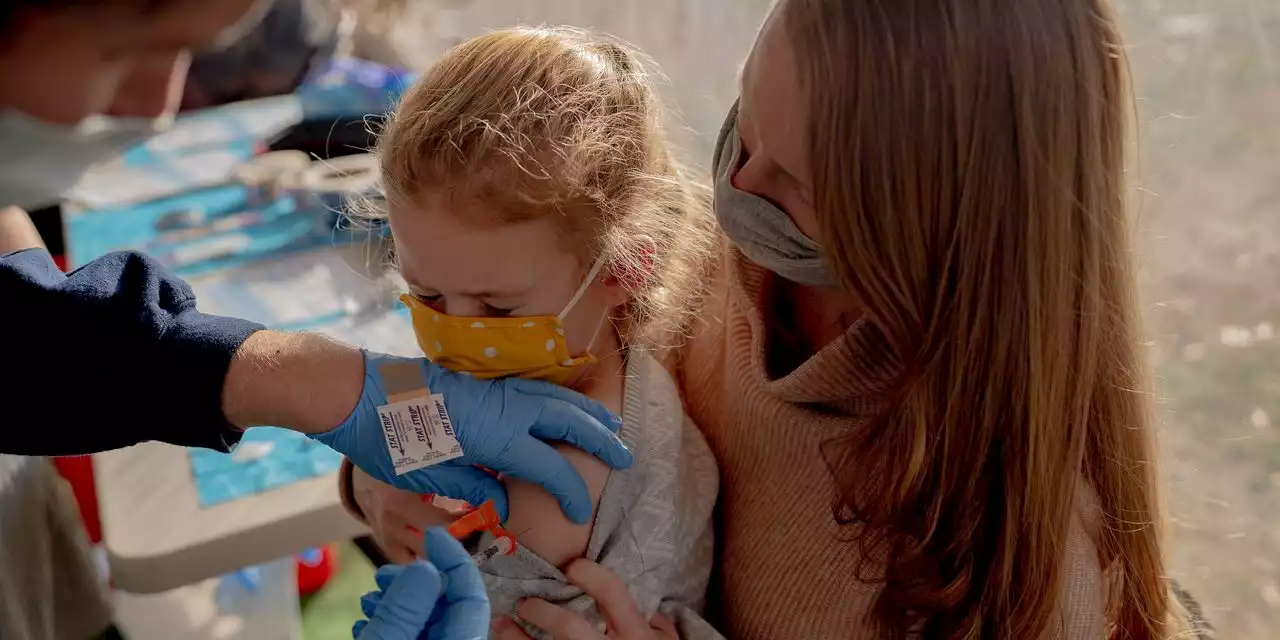 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Read more »
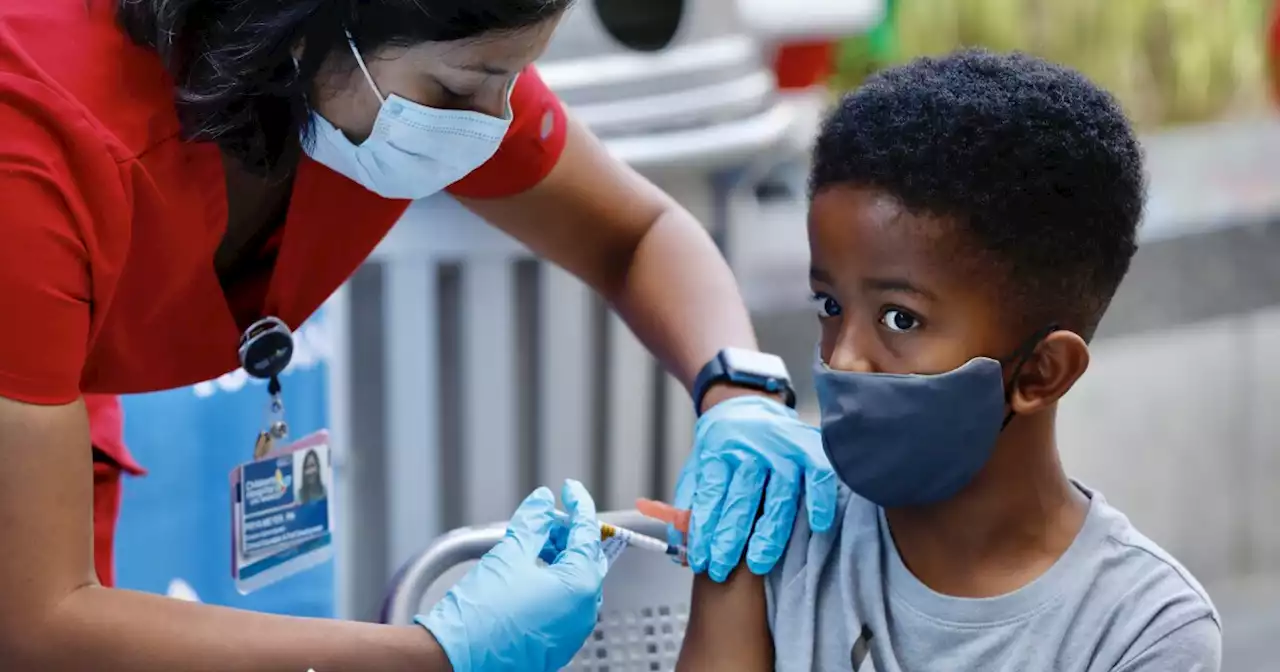 Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Pfizer asks FDA to clear COVID-19 booster shot for children ages 5 to 11Pfizer asked U.S. regulators for emergency-use authorization of a booster shot of its COVID-19 vaccine in children ages 5 to 11.
Read more »
 Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Read more »
 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Read more »
 Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Pfizer-BioNTech seeks FDA authorization for a COVID booster shot for kids 5-11Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
Read more »