Pfizer and German partner BioNTech are asking the FDA to authorize its omicron-targeting COVID-19 booster for school-age children.
at 15 micrograms to anyone 12 years old and older at least two months after any previous COVID-19 shots.
The companies said they planned to submit a similar application to the European Medicines Agency “in the coming days.”
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer and BioNTech seek authorization of omicron-targeted booster for children aged 5 to 11109.6 million people living in the U.S. have had a COVID booster, equal to 48.7% of the vaccinated population, the CDC’s tracker shows. Some 4.4 million people have had the new bivalent vaccines that target the latest omicron subvariants.
Pfizer and BioNTech seek authorization of omicron-targeted booster for children aged 5 to 11109.6 million people living in the U.S. have had a COVID booster, equal to 48.7% of the vaccinated population, the CDC’s tracker shows. Some 4.4 million people have had the new bivalent vaccines that target the latest omicron subvariants.
Read more »
 Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Read more »
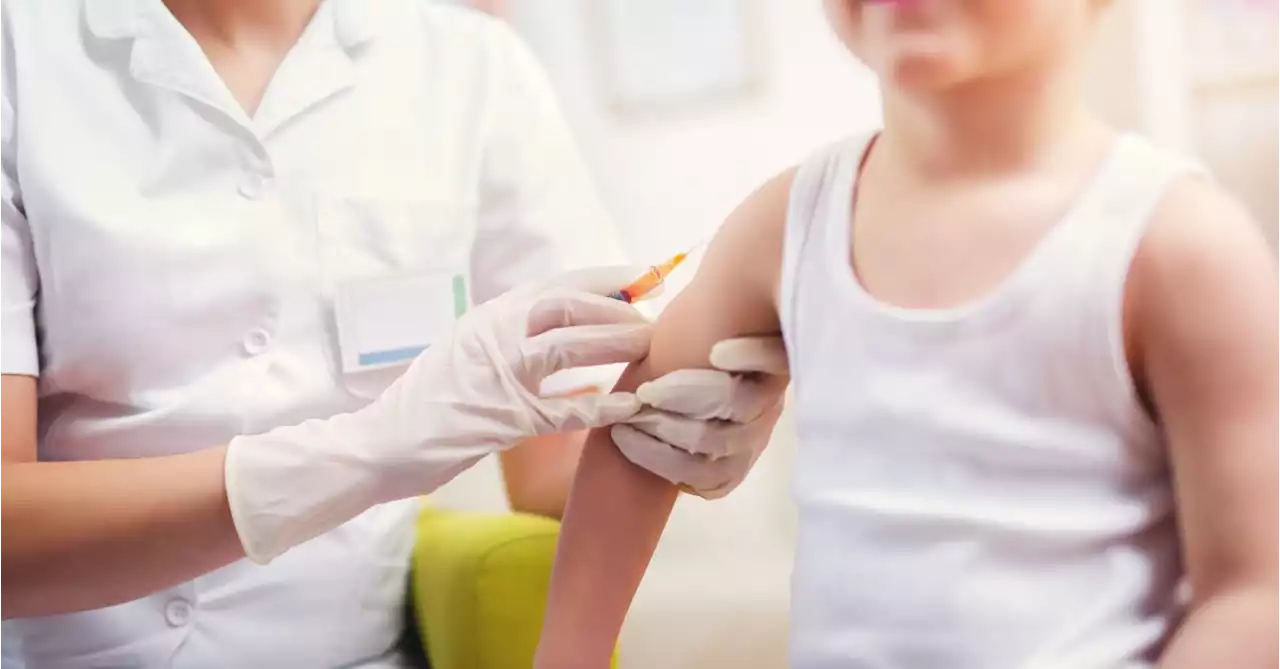 Moderna, Pfizer Seek Authorization for Children’s BoostersPfizer/BioNTech and Moderna, the two biggest COVID vaccine makers for the United States, are both seeking emergency authorization from the FDA for bivalent vaccine boosters for children.
Moderna, Pfizer Seek Authorization for Children’s BoostersPfizer/BioNTech and Moderna, the two biggest COVID vaccine makers for the United States, are both seeking emergency authorization from the FDA for bivalent vaccine boosters for children.
Read more »
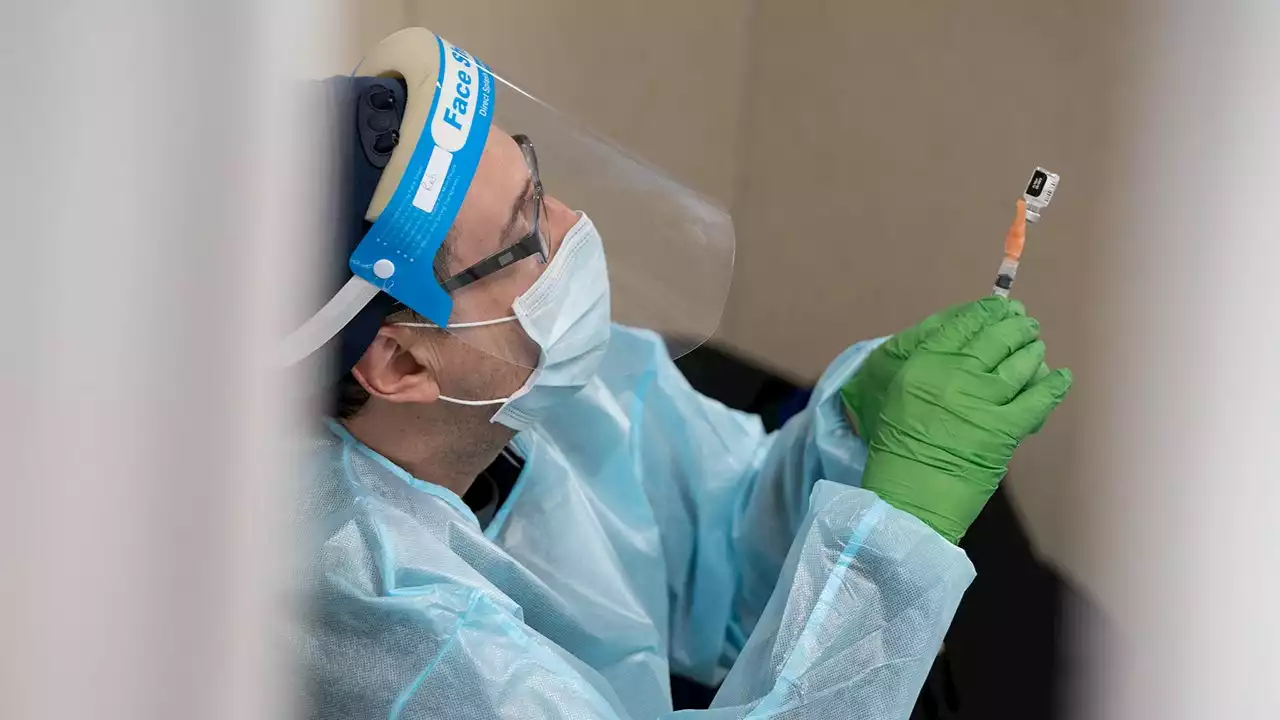 Bivalent booster: Pfizer seeks to expand shot to 5- to 11-year-oldsPfizer is asking the FDA to expand use of its updated COVID-19 vaccine booster shot to children ages 5 to 11. The so-called bivalent shot is meant to be more effective against omicron variants of the coronavirus.
Bivalent booster: Pfizer seeks to expand shot to 5- to 11-year-oldsPfizer is asking the FDA to expand use of its updated COVID-19 vaccine booster shot to children ages 5 to 11. The so-called bivalent shot is meant to be more effective against omicron variants of the coronavirus.
Read more »
 Pfizer submits application for updated COVID boosters for kids 5-11Both of the shots are bivalent, targeting the original 2019 strain as well as BA.4 and BA.5, which today account for 96% of new COVID-19 infections in the United States.
Pfizer submits application for updated COVID boosters for kids 5-11Both of the shots are bivalent, targeting the original 2019 strain as well as BA.4 and BA.5, which today account for 96% of new COVID-19 infections in the United States.
Read more »
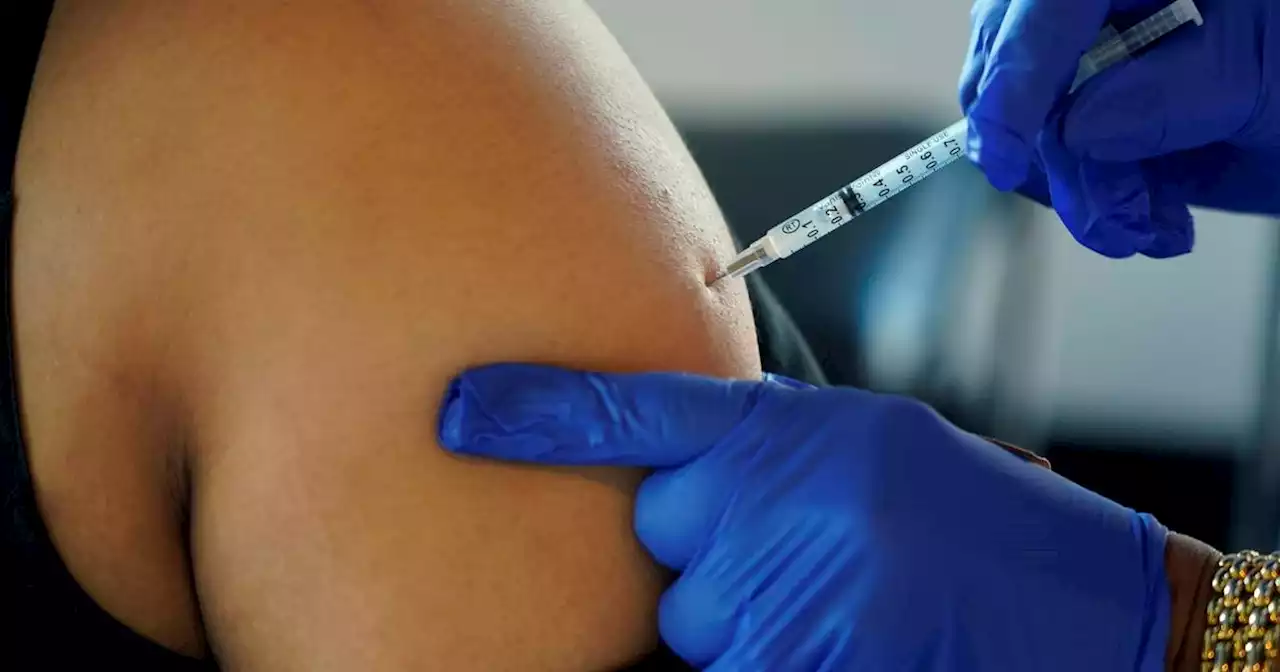 Pfizer seeks to expand omicron COVID booster to 5- to 11-year-oldsPfizer asked U.S. regulators Monday to expand use of its updated COVID-19 booster shot to children ages 5 to 11.
Pfizer seeks to expand omicron COVID booster to 5- to 11-year-oldsPfizer asked U.S. regulators Monday to expand use of its updated COVID-19 booster shot to children ages 5 to 11.
Read more »
