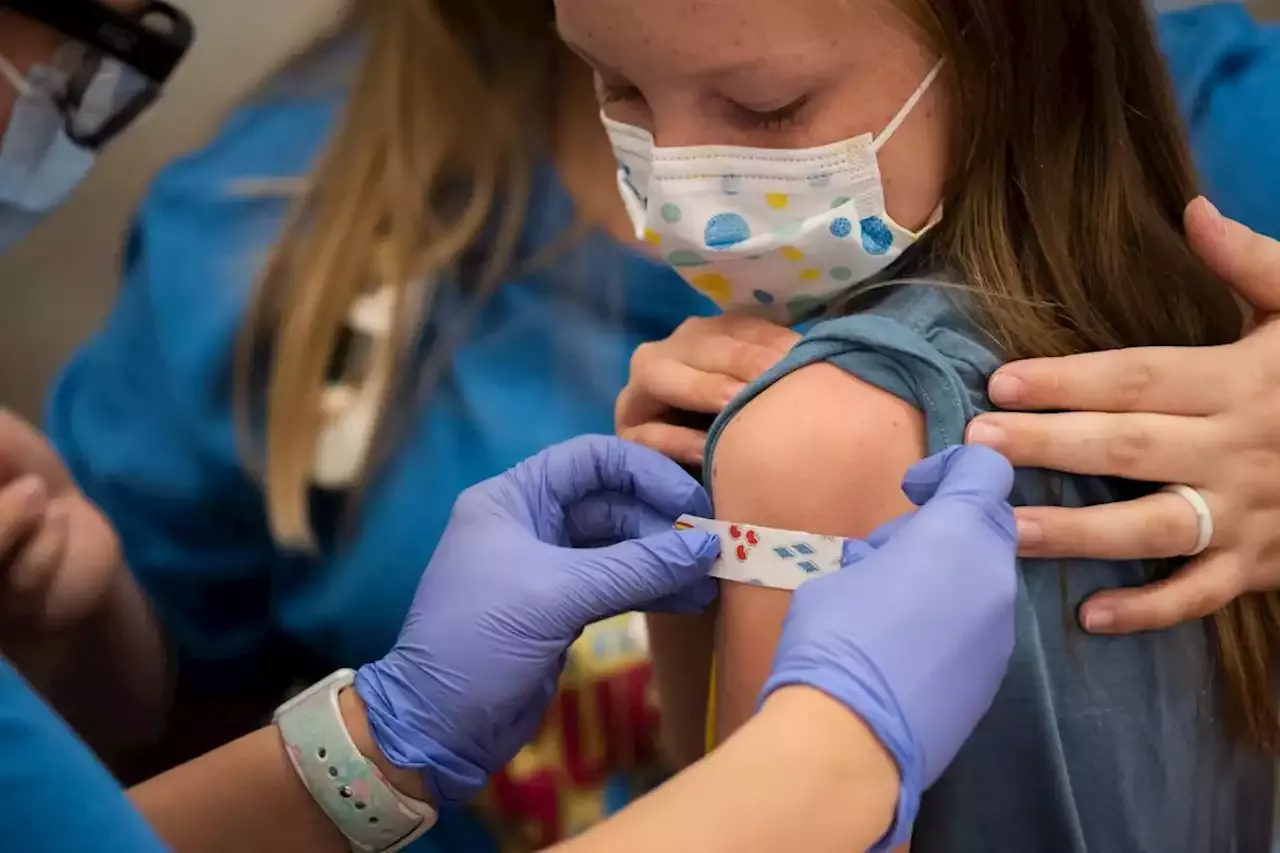
Pfizer and BioNTech have requested that the Food and Drug Administration authorize a COVID-19 booster shot for children ages 5-11.
The administration plans to increase NYPD spending by roughly $182 million, bringing the department’s budget to $5.6 billion and one of the most contentious fiscal issues back to the foreground.A generic antidepressant may be able to keep COVID-19 patients out of the hospital, though it’s unclear whether the cheap and readily available pill will be made part of the pandemic medicine cabinet.
The latest findings come from a review of three rigorous clinical trials conducted in Brazil, Canada, and the U.S. that points to a tie between fluvoxamine and a reduced risk of hospitalizations, according to the research, which was published April 6 in JAMA Network Open. The studies enrolled a total of 2,196 patients, though most of the data came from the Together trial, which had 1,497 participants.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
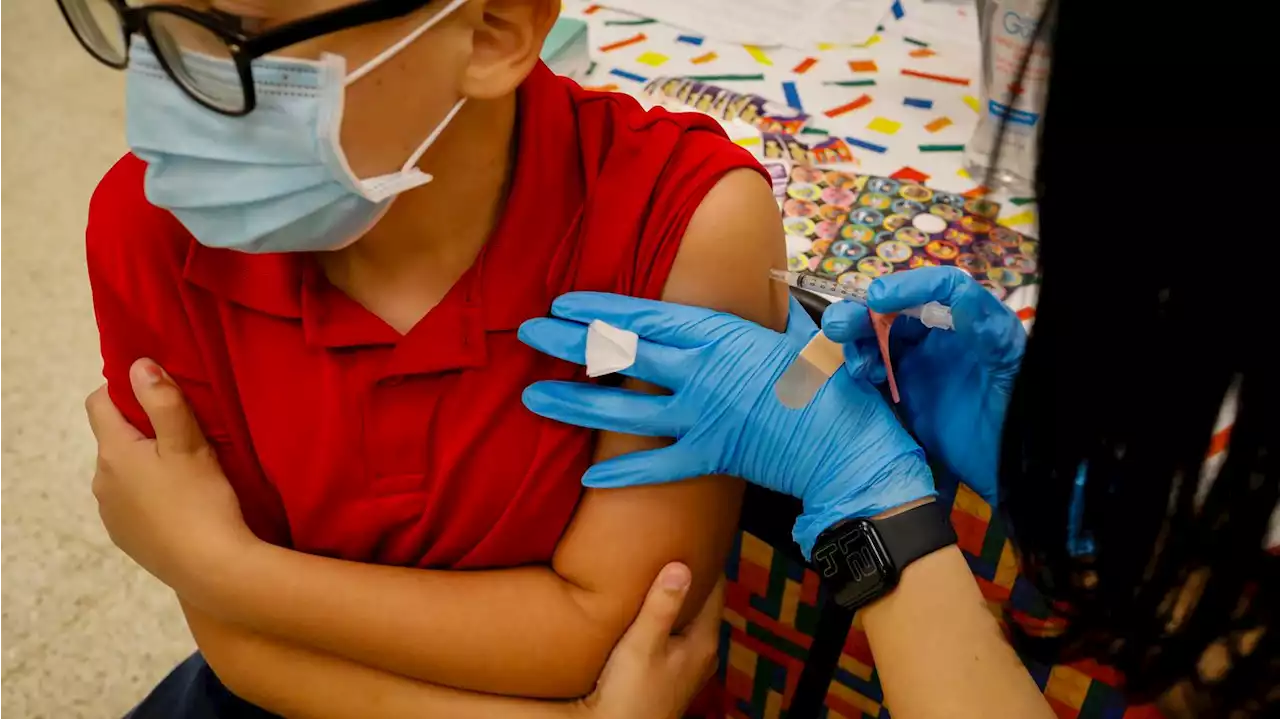 Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Pfizer asks FDA to authorize COVID booster for children 5 to 11If approved, it would be the first booster accessible to children of this age group, who were hit hard by Omicron.
Read more »
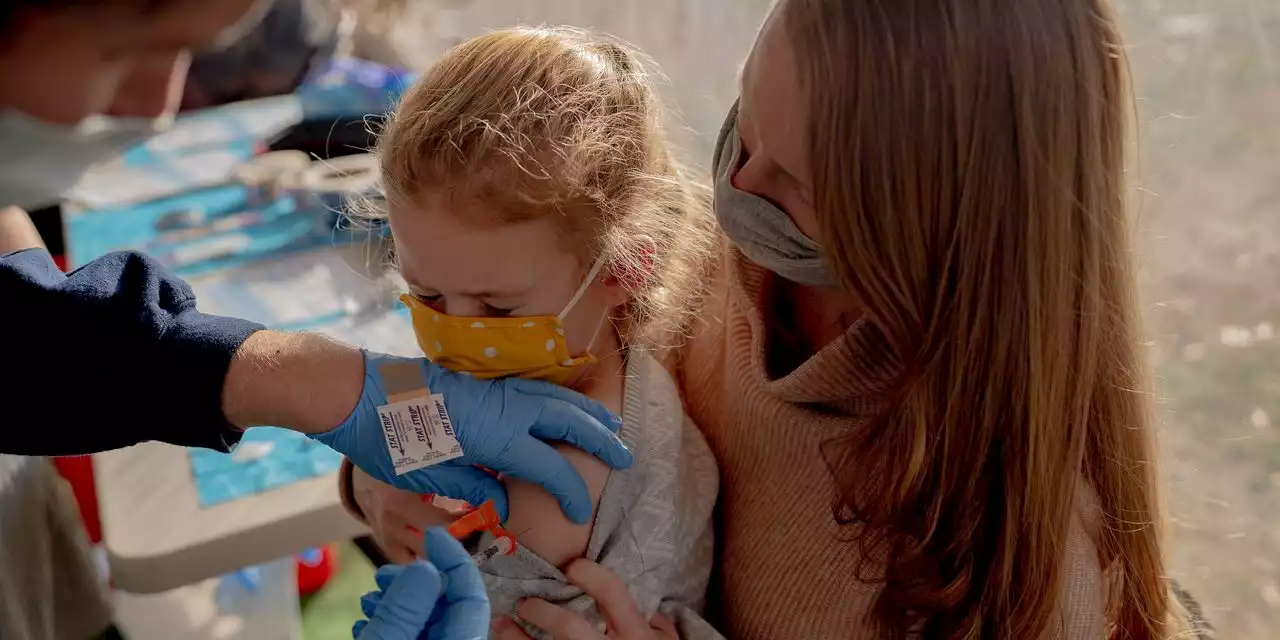 Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Pfizer Asks FDA to Authorize Covid-19 Booster for 5- to 11-Year-OldsPfizer Inc. and partner BioNTech SE said earlier this month that a third shot safely generated a strong immune response in the youngsters.
Read more »
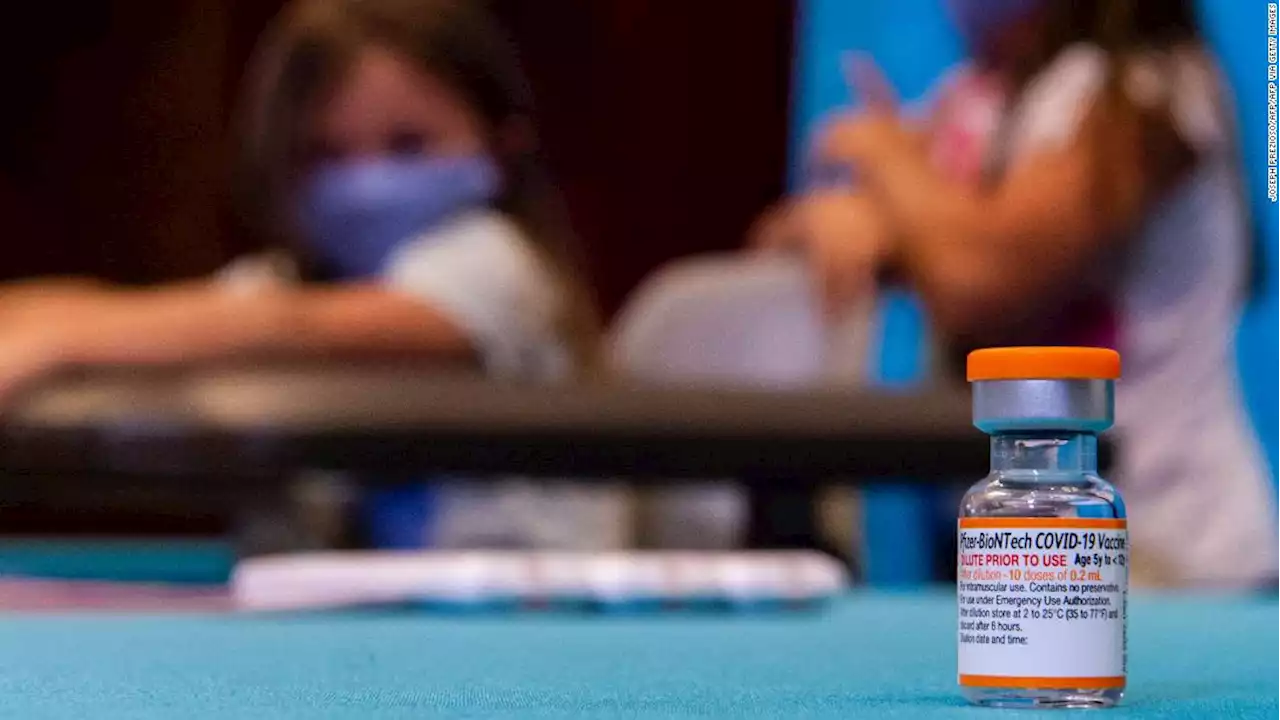 Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Pfizer requests FDA authorization for Covid-19 booster for kids 5 through 11Pfizer and BioNTech said Tuesday that they have submitted an application to the US Food and Drug Administration for emergency use authorization of a booster dose of Covid-19 vaccine for children 5 through 11.
Read more »
 Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Biden announces efforts to improve access to Pfizer COVID-19 treatmentThe Biden administration said it will attempt to save more lives with Pfizer’s effective treatment for COVID-19 by nearly doubling the number of pharmacies that stock the pills and setting up federally supported “test-to-treat” sites that offer the drug to people who test positive.
Read more »
 Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Pfizer Asks FDA to Authorize Third Dose for Children 5 to 11 Years OldPfizer on Tuesday asked the Food and Drug Administration to authorize a third dose of its COVID vaccine for children ages 5- to 11-years-old. The application comes after Pfizer released data earlier this month from a small lab study of blood samples from 30 kids in the age group, which showed a 36-fold increase in antibody levels against the omicron variant…
Read more »
 Pfizer Recalls Another Blood Pressure Medication
Pfizer Recalls Another Blood Pressure Medication
Read more »