New shot designed to protect babies against leading cause of hospitalization is expected to be available this fall.
A Pfizer Inc. PFE, +0.95% vaccine designed to protect babies from respiratory syncytial virus, or RSV, got a green light Monday from the U.S. Food and Drug Administration — a key milestone for infant health as well as for the pharmaceutical giant’s future growth strategy.
The regulatory approval for Pfizer’s shot, called Abrysvo, comes after a decades-long quest to shield infants from the virus, which has been recognized as a major cause of hospitalizations since the 1950s, Dr. William Gruber, Pfizer’s senior vice president for vaccine clinical research and development, told MarketWatch. The maternal shot is expected to be available in the third quarter of 2023.
Pfizer has said it could see peak sales of about $2 billion from its RSV vaccine, which is part of its broader respiratory-vaccine portfolio that the company expects will help drive future growth as it loses market exclusivity for some other major products in the coming years. A CDC advisory committee on immunizations earlier this month recommended that all babies under 8 months, born during or entering their first RSV season, get a dose of Beyfortus. Children between the ages of 8 months and 19 months who are at higher risk of severe RSV should also get a Beyfortus dose for their second RSV season, the CDC said.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
Read more »
 FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
Read more »
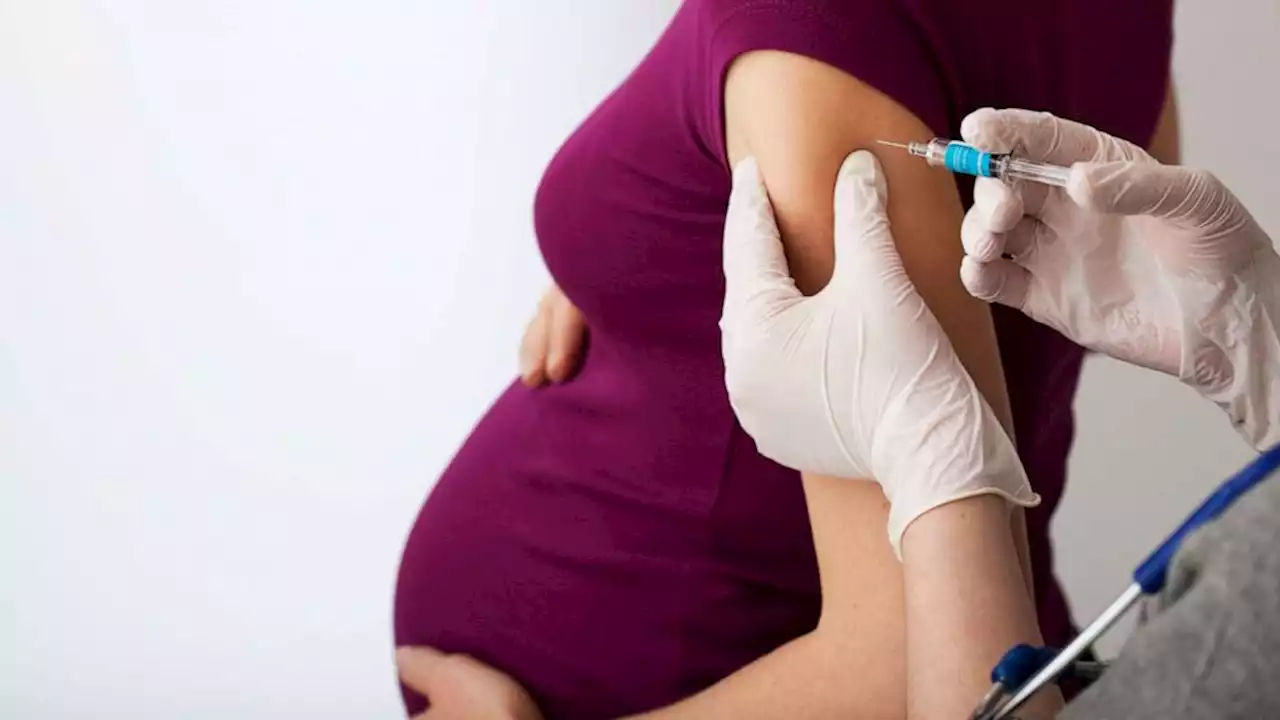 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Read more »
 FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
Read more »
 Flu, Covid and RSV: Preventing another 'Tripledemic'As summer nears its end and the school year begins, it's a good time to think about getting those fall vaccinations against viruses that circulate in colder weather. A so-called 'Tripledemic' of Covid-19, the flu and RSV spread throughout the country last winter. To prevent a repeat, health officials are encouraging everyone to get vaccinated against Covid-19 and the flu, and now there are new protections against RSV. CBS News Bay Area's Ryan Yamamoto asks Dr. Jorge Salinas, infectious diseases specialist at Stanford Health Care, about the recommended vaccines
Flu, Covid and RSV: Preventing another 'Tripledemic'As summer nears its end and the school year begins, it's a good time to think about getting those fall vaccinations against viruses that circulate in colder weather. A so-called 'Tripledemic' of Covid-19, the flu and RSV spread throughout the country last winter. To prevent a repeat, health officials are encouraging everyone to get vaccinated against Covid-19 and the flu, and now there are new protections against RSV. CBS News Bay Area's Ryan Yamamoto asks Dr. Jorge Salinas, infectious diseases specialist at Stanford Health Care, about the recommended vaccines
Read more »
