Pfizer asked U.S. regulators Monday to expand use of its updated COVID-19 booster shot to children ages 5 to 11.
Elementary school-aged children already received kid-sized doses of Pfizer’s original vaccine, a third of the dose given to everyone 12 and older — two primary shots plus a booster.
FDA vaccine chief Dr. Peter Marks said last week he expected a decision on boosters for that age group soon.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Pfizer CEO tests positive for COVID for a second timeAlbert Bourla had contacted the virus in August and took a course of the company's oral COVID-19 antiviral treatment, Paxlovid. He said he has not yet taken the new bivalent booster.
Pfizer CEO tests positive for COVID for a second timeAlbert Bourla had contacted the virus in August and took a course of the company's oral COVID-19 antiviral treatment, Paxlovid. He said he has not yet taken the new bivalent booster.
Read more »
 Pfizer CEO tests positive for COVID-19 for second time in two monthsPfizer CEO Albert Bourla announced Saturday that he tested positive for COVID-19 — again.
Pfizer CEO tests positive for COVID-19 for second time in two monthsPfizer CEO Albert Bourla announced Saturday that he tested positive for COVID-19 — again.
Read more »
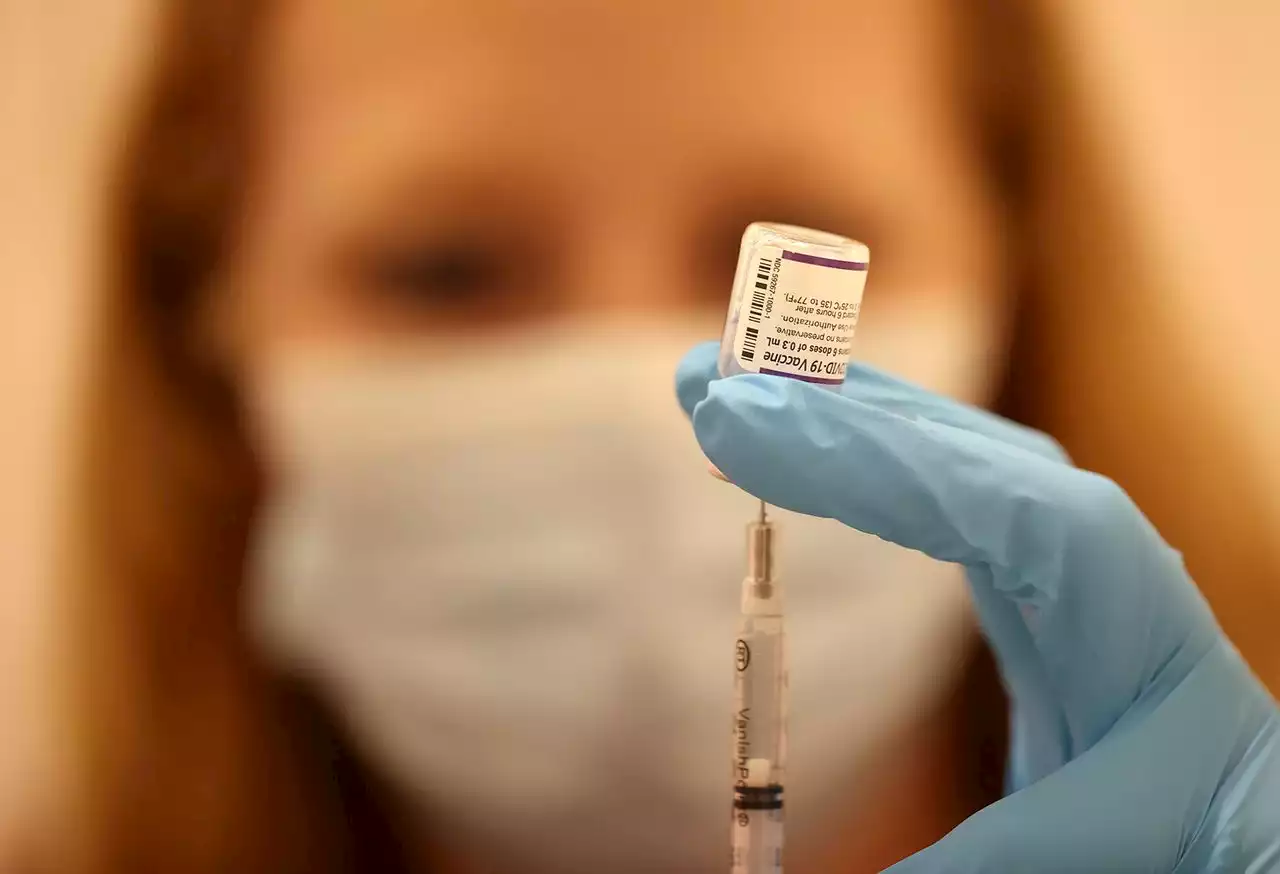 Pfizer seeks to expand COVID omicron booster to 5- to 11-year-oldsIf the FDA agrees, children would start getting a kid-sized dose of the new omicron-targeted formula.
Pfizer seeks to expand COVID omicron booster to 5- to 11-year-oldsIf the FDA agrees, children would start getting a kid-sized dose of the new omicron-targeted formula.
Read more »
 Pfizer Requests Clearance for Children’s Covid-19 BoosterPfizer and BioNTech asked U.S. health regulators to clear use of their updated Covid-19 booster in children 5 years to 11 years, just as retooled shots for people 12 and older roll out.
Pfizer Requests Clearance for Children’s Covid-19 BoosterPfizer and BioNTech asked U.S. health regulators to clear use of their updated Covid-19 booster in children 5 years to 11 years, just as retooled shots for people 12 and older roll out.
Read more »
 Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Pfizer Asks FDA to Authorize Omicron Covid Booster Shots for Kids Ages 5 to 11Pfizer’s application to the FDA comes before clinical trial results of the omicron BA.5 booster shots have been published.
Read more »
 Pfizer submits application for updated COVID boosters for kids 5-11Both of the shots are bivalent, targeting the original 2019 strain as well as BA.4 and BA.5, which today account for 96% of new COVID-19 infections in the United States.
Pfizer submits application for updated COVID boosters for kids 5-11Both of the shots are bivalent, targeting the original 2019 strain as well as BA.4 and BA.5, which today account for 96% of new COVID-19 infections in the United States.
Read more »
