Pfizer’s RSV vaccine transfers protective antibodies from mother to infant before birth.
Tamara Zangrilli was among the first people to take Abrysvo while pregnant — she signed up for Pfizer's clinical trial in 2021.
“If brought into broad use, there will be children who otherwise would have been hospitalized, otherwise would have ended up on ventilators this winter, that won’t,” said Dr. Bill Gruber, senior vice president of clinical research and development for Pfizer. Although the difference wasn’t statistically significant, the prescribing label for the vaccine will come with a warning not to administer Abrysvo before 32 weeks' gestation due to that numerical imbalance. Both of those observed rates were lower than thein the general population: around 10%, according to the CDC. Pfizer has said it will continue to monitor the risk of preterm birth among vaccine recipients.
The antibody injection, called Beyfortus, is CDC-recommended for babies up to 8 months old who are born during or entering their first RSV season, which typically starts around October. It’s also recommended for infants 8 to 19 months old who are at increased risk of severe disease and entering their second season.
Australia Latest News, Australia Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
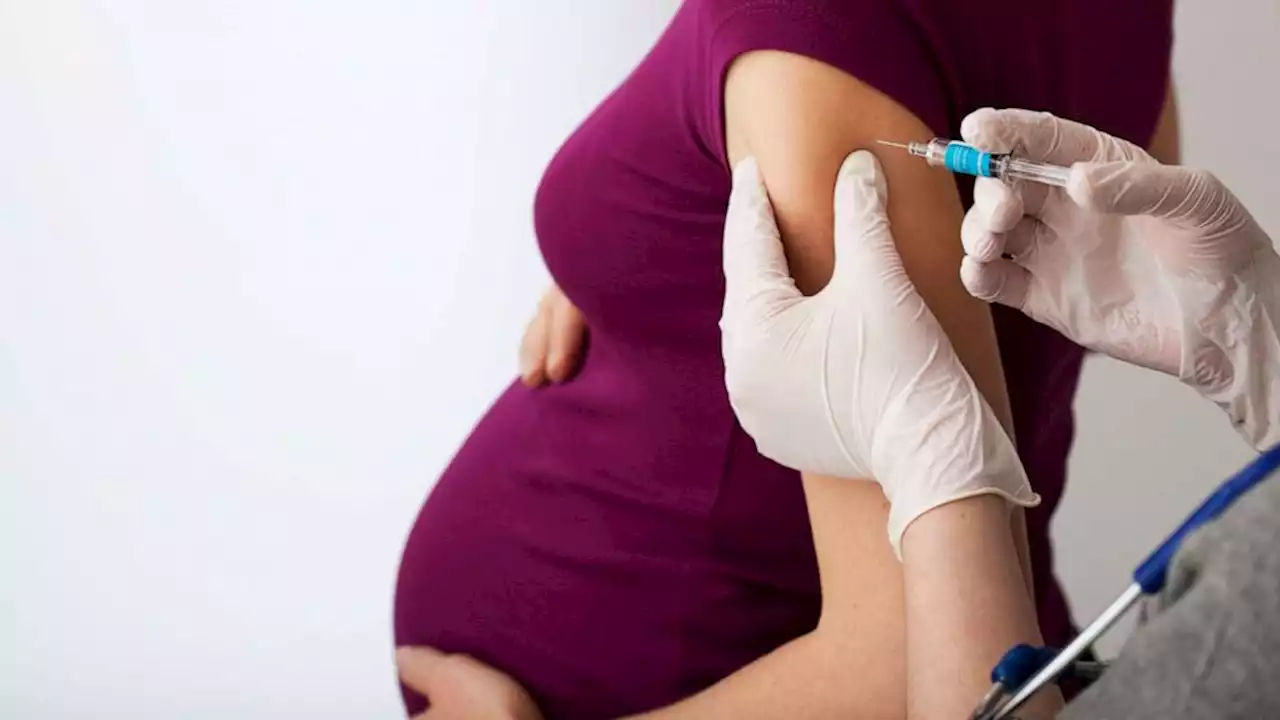 FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
FDA approves 1st maternal RSV vaccine to help protect infantsThe U.S. Food and Drug Administration has approved the first maternal vaccine intended to help protect newborns against respiratory syncytial virus (RSV).
Read more »
 US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
US FDA approves Pfizer's maternal RSV vaccine to protect infantsThe U.S. Food and Drug Administration on Monday approved Pfizer's respiratory syncytial virus (RSV) vaccine for use in women during the middle of the third trimester of pregnancy to protect their babies.
Read more »
 FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
FDA approves first RSV vaccine for pregnancy to protect newbornsExpectant parents could soon have another new option this fall to protect their newborns from RSV, the most common cause of hospitalization in American infants.
Read more »
 FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
FDA approves Pfizer maternal RSV vaccine for infantsPfizer hopes that its respiratory syncytial virus vaccine will be available to the public by the end of October or the beginning of November.
Read more »
 Pfizer maternal RSV vaccine gets FDA approvalNew shot designed to protect babies against leading cause of hospitalization is expected to be available this fall.
Pfizer maternal RSV vaccine gets FDA approvalNew shot designed to protect babies against leading cause of hospitalization is expected to be available this fall.
Read more »
